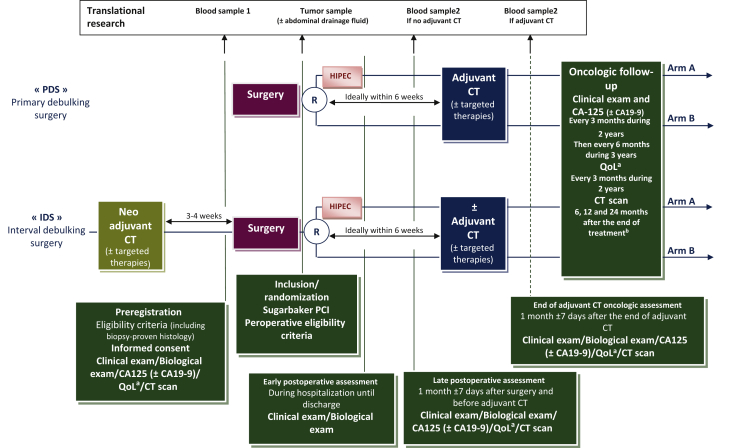
Figure 1.
The CHIPPI trial design.
The enrollment starts with a preregistration prior to surgery and a confirmation of enrollment and randomization during surgery. The experimental arm (arm A) involves the addition of HIPEC with the standard of care (chemotherapy and cytoreductive surgery). The control arm (arm B) consists of the standard of care alone (chemotherapy and cytoreductive surgery). Adjuvant or neoadjuvant chemotherapy regimens are associated or not with bevacizumab and PARP inhibitors. Adjuvant chemotherapy is preferably started within the first 6 weeks following surgery. Postoperative assessment starts immediately after surgery, is reassessed at 1 month after surgery before starting adjuvant chemotherapy and 1 month after achieving adjuvant chemotherapy. Patients will undergo physical examination with evaluation of tumor markers if informative (CA125 and/or CA19-9) every 3 months for 2 years, and then every 6 months until achieving 5 years of follow-up. Patients will also undergo CT scan imaging at 6, 12 and 24 months after achieving their treatment. Quality of life assessment will be carried out every 3 months for 2 years.
CT, computed tomography; EORTC, European Organization for Research and Treatment of Cancer; HIPEC, hyperthermic intraperitoneal chemotherapy; PCI, Peritoneal Cancer Index; QLQ-C30, Quality of Life Questionnaire-Score 30; QLQ-OV28, Quality of Life Questionnaire-Ovarian Cancer Module.
a Quality of life assessed by EORTC QLQ-C30 and QLQ-OV28 questionnaires.
b End of treatment is defined as the last day of adjuvant CT excluding targeted therapies or as the date of surgery if no adjuvant CT is administered.