Figure 4.
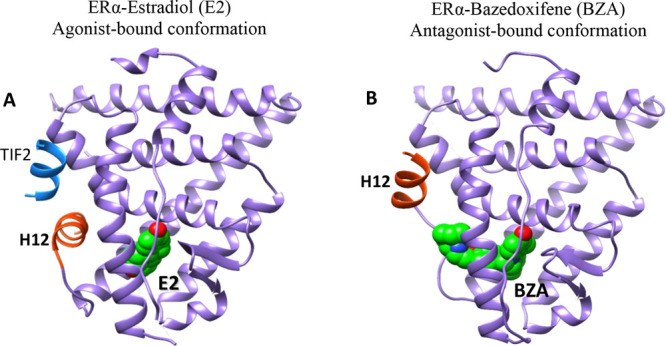
Comparison of the crystal structures of ERα in active (agonist-bound) and inactive (antagonist-bound) conformations. (A) Active form when bound to Estradiol (E2) and a short peptide from TIF2 transcriptional coactivator bearing canonical LXXLL motif (PDB code: 1GWR) and (B) inactive form when bound to bazedoxifene (BZA) (PDB code: 4XI3). In the antagonist-bound conformation, H12 is repositioned to occupy the coactivator binding groove.
