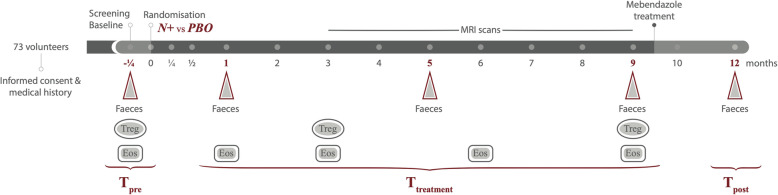
Fig. 1.
Overview of the clinical study design. A total of 73 volunteers suffering from relapsing multiple sclerosis (RMS) were included in the trial and randomly assigned to the two treatment arms, i.e. percutaneous infection with 25 N. americanus larvae (N+), or placebo treatment with pharmacopoeial grade water (PBO). Triangles indicate the timepoints corresponding to faecal sample collection for metagenomic sequencing, whilst ellipses and rectangles indicate collection of blood samples for assessment of regulatory T cell (Treg) and eosinophil (Eos) counts, respectively (data available from [10]). Samples collected prior to hookworm infection = Tpre; samples collected at 1, 5, and 9 months post-infection/placebo treatment = Ttreatment; samples collected post-anthelmintic treatment = Tpost