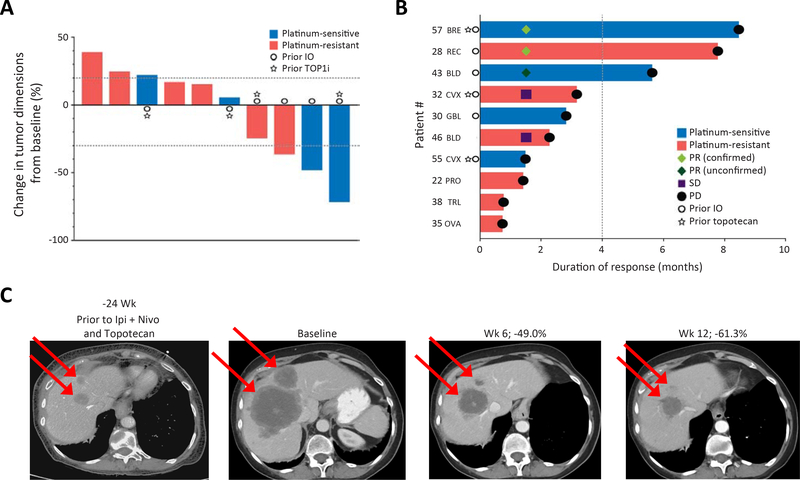
Figure 4: Efficacy of M6620 and topotecan in SCNCs regardless of tissue of origin.
A) Responses to the combination in EP-SCNCs based on change in tumor dimensions from baseline. Each bar represents a patient’s tumor response. The top and bottom dotted lines indicate the cutoffs of disease progression and partial response per RECIST criteria, respectively. B) Efficacy in EP-SCNCs based on duration of response. The dotted line indicates the 4-month timepoint after treatment. C) CT abdomen showing tumor regression in a patient with breast SCNC and liver metastases (patient #57). The patient had undergone extensive previous treatments including topotecan. REC, rectal; BLD, bladder; TRL, transformed lung cancer; BRE: breast; CVX, cervix; GBL, gall bladder; PRO, prostate; OVA, ovarian. See also Table S3.