Figure 5.
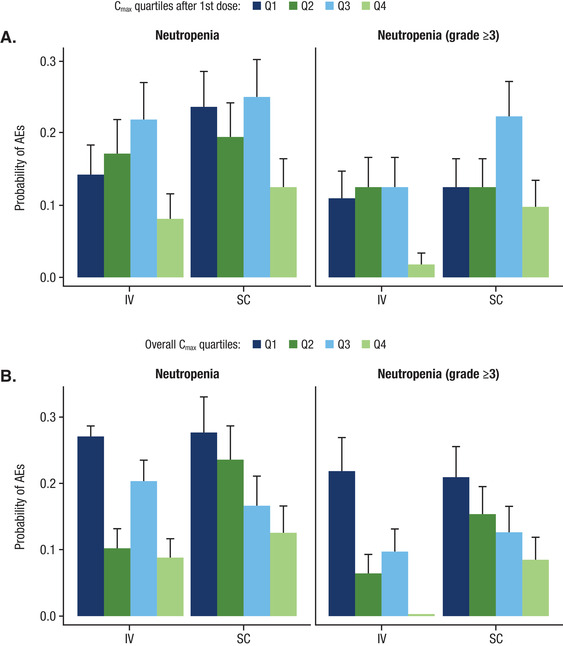
Rate of any grade and grade ≥3 neutropenia in relation to (A) Cmax after the first dose and (B) overall Cmax after subcutaneous DARA 1800 mg or intravenous DARA 16 mg/kg monotherapy. Cmax, maximum peak concentration; DARA, daratumumab; Q, quartile; AE, adverse event.
