Scheme 2.
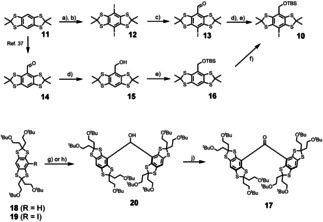
Synthesis of building blocks 10 and 17. a) 6.5 equiv. LiTMP, 0.1 equiv. Et3NHCl, 12 equiv. Me3SiCl, THF, −95 °C to r.t., 16 h. b) 3.0 equiv. ICl, CH2Cl2, r.t., 3 h, 86 % over two steps. c) 1.1 equiv. nBuLi, THF, −95 °C, 45 min, then 15.0 equiv. DMF, to r.t., 16 h. 73 % yield. d) 2.0 equiv. NaBH4, CH2Cl2/MeOH 2:1, r.t., 30 min, 93 % from 14. e) 1.2 equiv. tBuMe2SiCl, 2.5 equiv. Imidazole, DMF, r.t., 16 h, 82 % over two steps from 13, 91 % from 15. f) 1. LiTMP, THF, −78 °C, 2 h. 2. I2, to r.t., 16 h, 56 %. g) 0.95 equiv. nBuLi, THF, −95 °C, then 0.4 equiv. HCO2Me, r.t., 16 h, 36 %, for 12. h) 1.95 equiv. tBuLi, 0.48 equiv., THF, −95 °C, 45 min, then 0.48 equiv. HCO2Me, r.t., 16 h, 88 %, for 13. i) 1.25 equiv. Dess–Martin‐periodinane, CH2Cl2, r.t., 60 min, 93 %.
