Scheme 2.
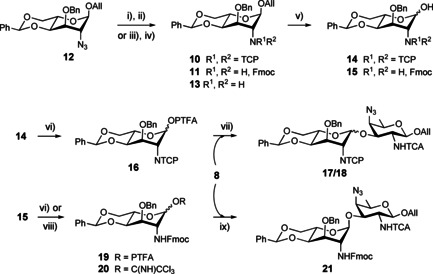
[A+B] glycosylation by use of a 4,6‐O‐benzylidene A donor. (i) Zn, AcOH, THF, (ii) a. TCPO, Et3N, DCE, 50 °C, b. Ac2O, py, 90 °C, 83 % over 2 steps, (iii) PPh3, H2O, THF, 60 °C, (iv) FmocCl, NaHCO3, DMAP, DCM, 0 °C, 88 % over 2 steps, (v) a. H2‐activated Ir‐cat, THF, b. I2, NaHCO3, THF/H2O, 43 % for 14, and NIS, THF/H2O, 90 % for 15, (vi) PTFA‐Cl, Cs2CO3, Acet, 80 % for 16, 90 % for 19, (vii) TMSOTf, MS 4 Å, DCE, −30 °C, 80 %, (viii) CCl3CN, K2CO3, Acet, quant., (ix) TMSOTf, MS 4 Å, DCE, −15 °C, 62 % (from crude donor 20). Acet: acetone, Ir‐cat: [Ir(COD)(PMePh2)2]PF6. PTFA: (N‐phenyl)trifluoroacetimidoyl.
