Scheme 4.
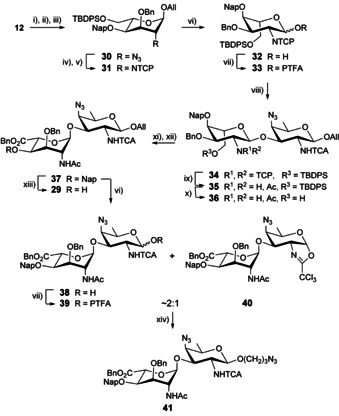
Synthesis of the AB azidopropyl glycoside 41 by means of a [A+B] glycosylation using the orthogonally protected A donor 33. (i) CSA, MeOH/DCM (4:1, v/v), (ii) TBDPSCl, Imidazole, DMF, (iii) NapBr, NaH, DMF, 0 °C, 85 % over 3 steps, (iv) Zn, AcOH, THF, (v) a. TCPO, Et3N, DCM, b. Ac2O, Py, 80 °C, 82 % over 2 steps, (vi) a. H2‐activated Ir‐cat, THF, b. NIS, THF/H2O, 82 % for 32, 92 % for 38, (vii) PTFA‐Cl, Cs2CO3, Acet, quant. for 33, 89 % for 39/40 (∼2:1), (viii) 8, TMSOTf, DCM, −15 °C, 96 %, (ix) a. Ethylenediamine, MeOH/THF (1:1, v/v), 50 °C, b. Ac2O, MeOH, 94 %, (x) TBAF, THF, 86 %, (xi) TEMPO, BAIB, DCM/H2O, (xii) BnBr, K2CO3, DMF, 85 % over 2 steps, (xiii) DDQ, DCM/Phosphate buffer pH 7 (6:1, v/v), 0 °C to rt, 87 %, (xiv) 3‐Azidopropanol, Yb(OTf)3, DCM, 0 °C, 78 %.
