Figure 6. Administration of the Histone Deacetylase Inhibitor TSA Reverses the Recognition Memory Deficit in CBP{HAT‒} Mice.
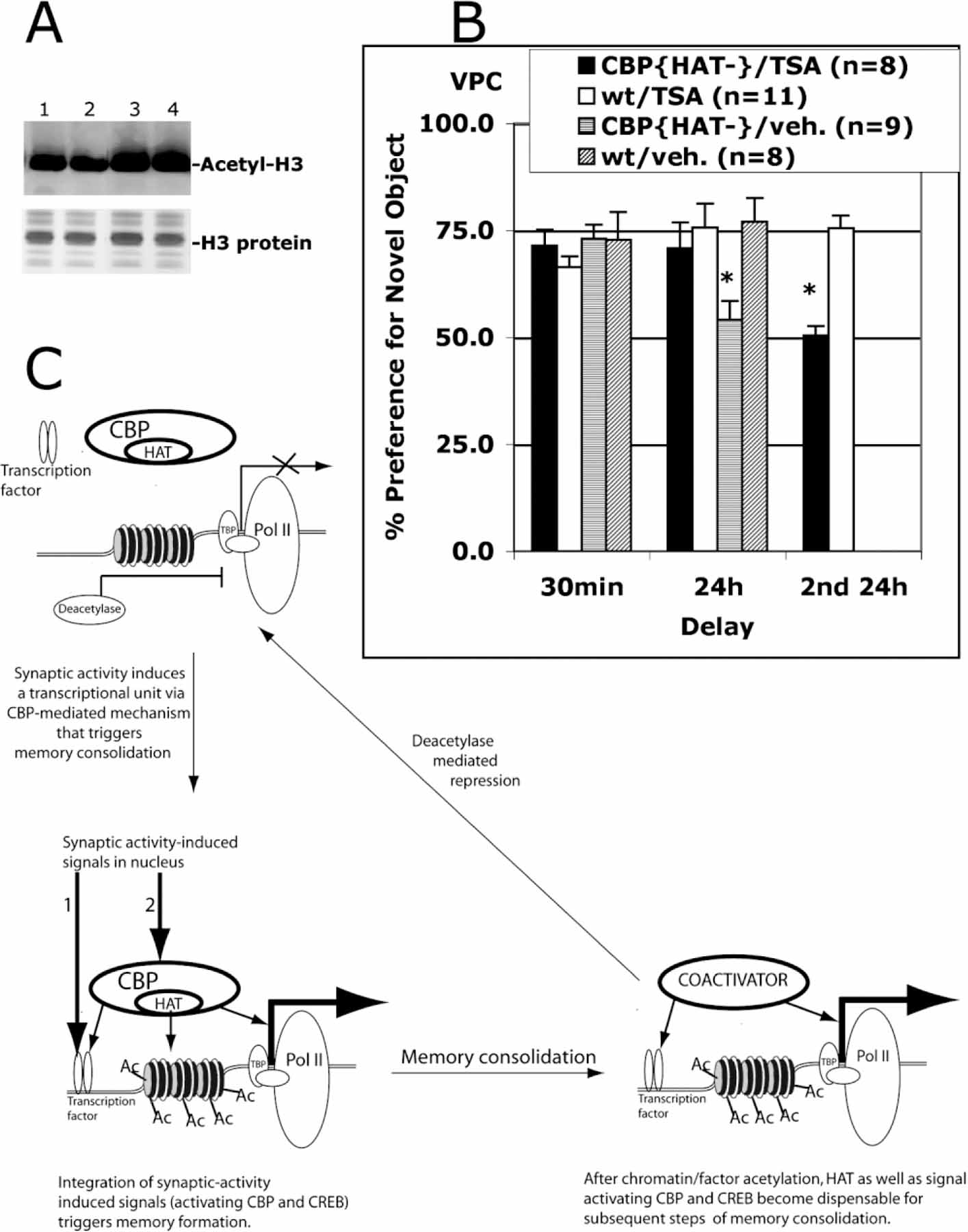
(A) I.p. TSA injection increases histone acetylation levels in the hippocampus. Nuclear extracts were prepared from hippocampi isolated from wt mice sacrificed 6 hr after i.p. injection of vehicle (lanes 1 and 2) or TSA (lanes 3 and 4). Acid-extracted histones were visualized with Coomassie blue staining. The level of histone H3 acetylation was assessed in Western blot assay using anti-acetylated H3 antibodies (Upstate).
(B) I.p. TSA administration before training rescued a recognition memory deficit in CBP{HAT‒} mice. Four groups of mice were tested: CBP{HAT‒}/TSA (Group 1), wt/TSA (Group 2), CBP{HAT‒}/veh (Group 3), and wt/veh (Group 4). TSA or DMSO alone (veh) was administered 2 hr before training on the VPC task as described in Experimental Procedures. Then, we measured performance of these four groups on the VPC task at 30 min and 24 hr delays. 4 days later, the recognition memory was assessed again (Groups 1 and 2) on the VPC task at the 24 hr delay using a new set of objects (2nd 24 hr). This assay provided a control showing that Group 1 has strong memory impairment if TSA was not administered.
(C) Model of acetylation-dependent memory consolidation. The proposed model takes into account five observations: (1) NMDAR-dependent neuronal activation is required for memory formation (Davis et al., 1992); (2) neuronal activity-dependent transcription is required for memory consolidation (Andrew, 1980; Davis and Squire, 1984); (3) NMDA-dependent transcription requires a signaling pathway to activate transcription factors such as CREB and a separate signaling pathway to activate CBP (Chawla et al., 1998; Impey et al., 2002); (4) behaviorally induced CREB phosphorylation is transient and does not correlate with peak Fos induction (Stanciu et al., 2001); and (5) CBP acetyltransferase activity is required for long-term memory consolidation but not for short-term memory (our data). This model suggests that CBP-mediated histone acetylation during transcriptional activation is a limiting step in the molecular mechanism controlling memory stabilization. Initial steps include induction of transient CREB phosphorylation, CBP activation, and CBP-mediated histone acetylation at a specific transcriptional unit in response to the initial learning event. Subsequently, prolonged elevated transcription required for memory consolidation could be maintained by CBP- and CREB phosphorylation-independent nuclear mechanisms even after signals to CREB and CBP are no longer present. This ongoing transcription would remain active until the competing deacetylase-dependent repression mechanism shut off transcription.
