Figure 1 |. Cross-coupling reactions using configurationally stable, optically active secondary alkyl nucleophiles.
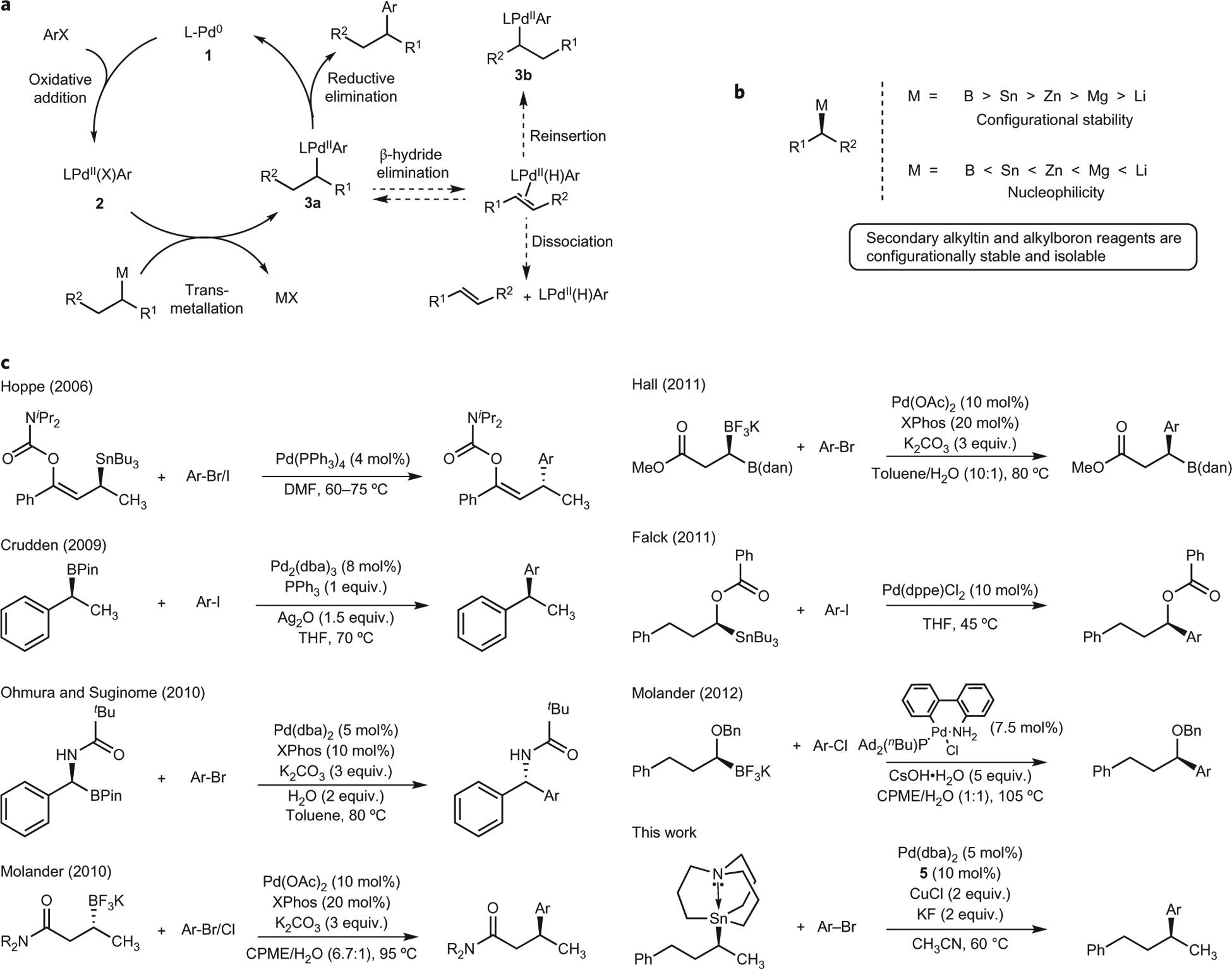
a, Proposed catalytic cycle for the Pd-catalysed cross-coupling of secondary nucleophiles and aryl electrophiles. It is necessary for reductive elimination to occur in the absence of the competing β-hydride elimination pathway to avoid the formation of isomeric by-products. b, Inverse relationship between configurational stability and nucleophilicity in secondary alkyl organometallic nucleophiles. c, Recent advances in Pd-catalysed cross-coupling reactions between isolable, optically active organometallic nucleophiles and aryl halides. The groups of Hoppe29, Crudden22, Ohmura and Suginome32, Molander31,37, Hall35 and Falck28 have demonstrated the use of isolable, optically active organometallic nucleophiles in cross-coupling reactions. However, nucleophiles in these examples require activation via the presence of a C(sp2) α-carbon, heteroatomic α-carbon and/or a strongly coordinating β-carbonyl group. DMF, dimethylformamide; THF, tetrahydrofuran; CPME, cyclopentyl methyl ether; dba, dibenzylideneacetone; XPhos, 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl.
