FIGURE 3.
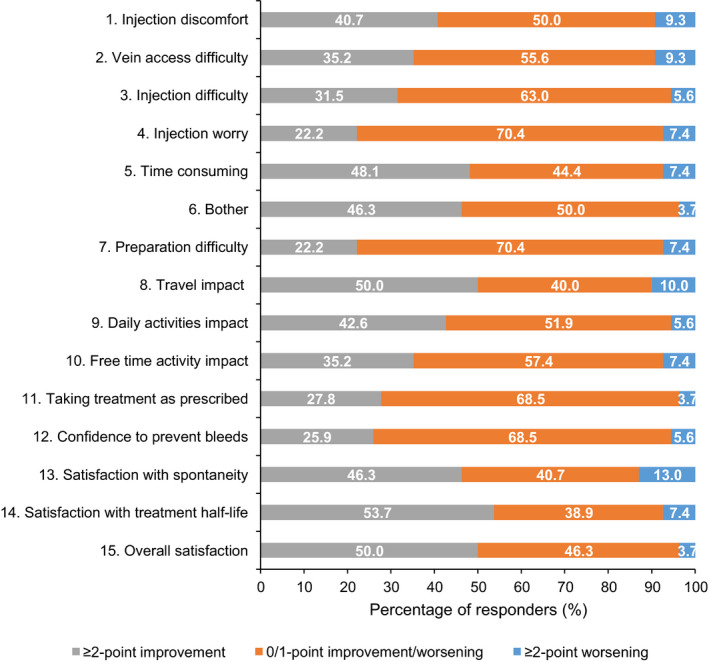
Shift from baseline in scores by ≥2‐point improvement or worsening to follow‐up analysis† for SQ‐ISHI items. ≥2‐point improvement is considered clinically relevant. †Follow‐up analysis: data included from the original Week 21 assessment if available or the original Week 25 if available and original Week 21 not available. If both Week 21 and Week 25 assessments were missing, then it was set as missing. ‡Participants could answer ‘not applicable’ for travel impact. Abbreviations: SQ‐ISHI, Satisfaction Questionnaire—Intravenous Subcutaneous Hemophilia Injection
