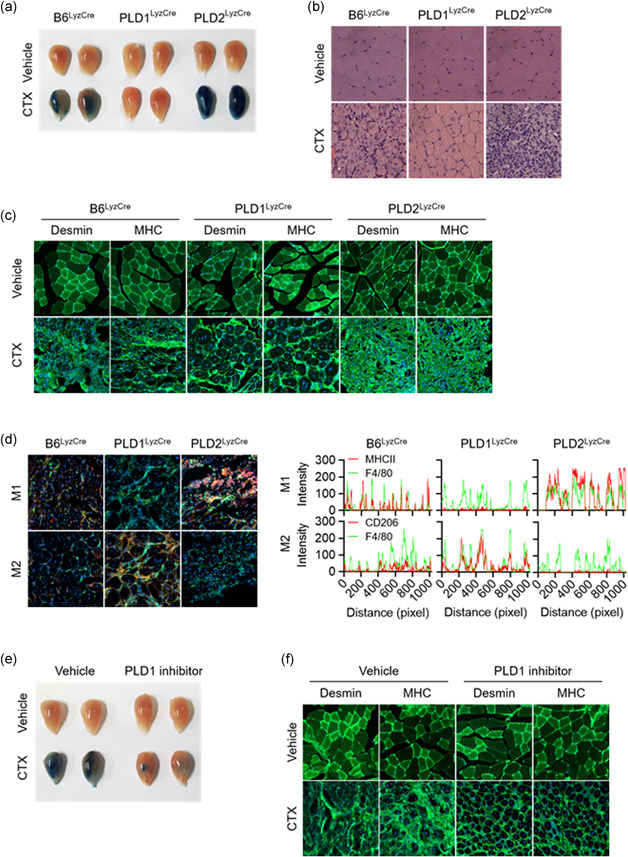
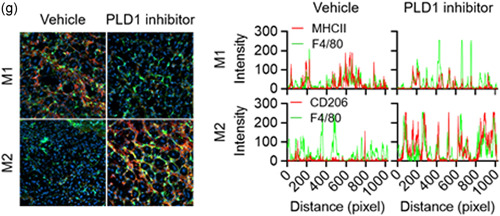
Figure 5.
PLD1 ablation in macrophages promotes muscle regeneration by regulating the phenotype switch of muscle macrophages from M1 to M2. (a) Representative photograph of TA muscles of the indicated mice after 8 days of injury by cardiotoxin (CTX) (n = 8 mice per group). (b) Representative images of TA muscle sections stained with hematoxylin and eosin after 8 days of injury. (c) TA muscles of the specific mice were stained with the indicated antibodies and observed by fluorescence microscopy, 5 days after injury. (d) TA muscles of the indicated mice were stained with Hoechst 33342 (blue) and antibodies to F4/80 (green), MHCII (red, I‐Eb/I‐Ab), or CD206 (red) and observed by fluorescence microscopy, 5 days after injury. Fluorescence intensity profiles of the F4/80‐positive signal (green) and MHCII (red) or CD206 (red)‐positive signal showing that the two signals share overlapping spatial profiles. (e) Representative photograph of TA muscles of PLD1 inhibitor‐injected mice after CTX injury (n = 8 mice per group). (f) PLD1 inhibitor‐injected TA muscles after CTX injury were stained with the indicated antibodies and (g) stained with Hoechst 33342 (blue), and antibodies to F4/80 (green), MHCII (red), or CD206 (red). The images were observed by fluorescence microscopy. Fluorescence intensity profiles of the F4/80‐positive signal (green) and MHCII (red) or CD206 (red)‐positive signal. Results are representative of at least five independent experiments and presented as the mean ± SD. Magnification is ×200. PLD, Phospholipase D; TA, tibialis anterior