Figure 2.
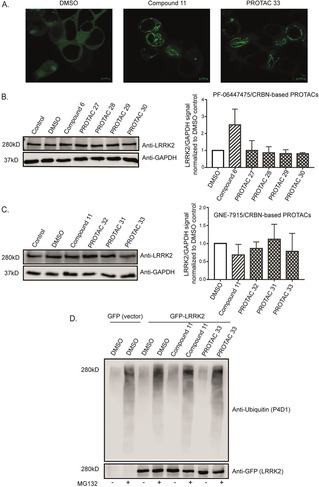
PROTACs are cell‐permeable yet not increasing LRRK2 degradation and ubiquitination. A) Confocal microscopy of GFP‐tagged LRRK2‐transfected HEK293 cells showing LRRK2 localization inside cells treated with DMSO, original kinase inhibitor and one of the PROTACs. The images show that the original kinase inhibitor and the PROTAC compound are cell‐permeable and able to induce LRRK2 localization to the microtubules depicted as green filaments. B) and C) Representative western blots of LRRK2 parental RAW 264.7 cells treated with 10 μM of different PROTACs together with the original kinase inhibitors and DMSO controls for 24 h and immunoblotted with LRRK2 and GAPDH as a loading control. The quantification of the blots (n=3) shows that different PROTACs are not affecting LRRK2 degradation compared to the original kinase inhibitor.. D) GFP‐tagged LRRK2 transfected HEK293 cells were treated with DMSO, original kinase inhibitor (11) and PROTAC (33) with or without MG132 for 24 h. An ubiquitin assay was performed on the collected cells. The samples were immunoblotted with anti‐ubiquitin (P4D1) for the ubiquitin signal and anti‐GFP for the LRRK2 signal. The blot shows that MG132 can enhance the ubiquitin signal and that neither the original kinase inhibitor (11) nor PROTAC (33) increase LRRK2 ubiquitination compared to the DMSO treated control.
