Scheme 1.
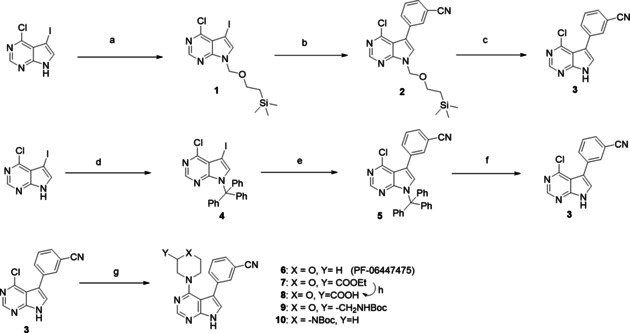
Synthetic routes to reach main intermediate (3) for PF‐06447475‐based PROTACs; original (top), modified (middle). Bottom: transformations to intermediates with functional groups. Reagents and conditions: a) NaH, SEM‐Cl, THF, 0 °C to RT, 3 h, 40 % yield; b) (3‐cyanophenyl)boronic acid, 1 % Pd(dppf)Cl2, K2CO3, DME/H2O, reflux 3 h, 30 % yield; c) TFA, RT, 24 h, 60 % yield; d) trityl chloride, CHCl3, Et3N, RT, 1 h, quantitative; e) (3‐cyanophenyl)boronic acid, 0.004 % Pd(dppf)Cl2, NaHCO3, toluene/EtOH, reflux 24 h, 60 % yield; f) TFA, CH2Cl2, RT, 24 h, 90 % yield; g) morpholine for (6) tert‐butanol, DIPEA, reflux 3 h, or ethyl morpholine‐2‐carboxylate for (7) or tert‐butyl N‐(morpholin‐2‐ylmethyl)carbamate for (9) or N‐Boc‐piperazine for (10), EtOH, DIPEA, MW, 150 °C, 1 h, yields 26–30 %; h) LiOH, THF/H2O, 65 % yield.
