Figure 2.
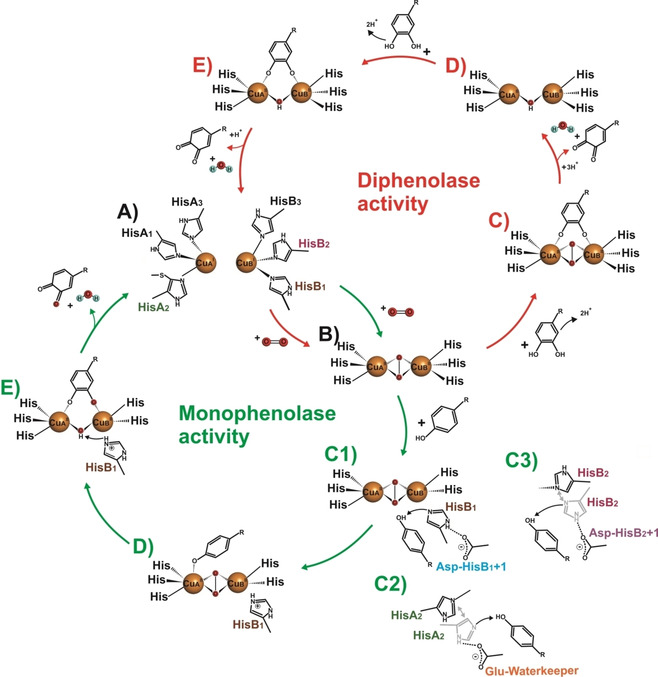
Schemes of the mono‐(green cycle) and diphenolase (red cycle) activity of PPOs. Α) The deoxy‐form of the type‐III copper center (CuI‐CuI) is the starting point for both activities. It binds molecular oxygen and thereby switches to the catalytically active oxy‐form (CuII‐CuII). Β) Monophenolase activity (green): C1), C2), C3) residues located within or around the dicopper center (HisB1+1, HisB2+1, and the waterkeeper residue) enhance the basicity of the conserved copper‐coordinating histidines (HisB1, HisB2, and HisA2), which then deprotonate the incoming monophenolic substrates. D) The deprotonated monophenol, ready for the catalytic reaction, interacts with the oxy‐form of the type‐III copper center. E) ortho‐hydroxylation of the phenolate by an electrophilic aromatic substitution and the subsequent two‐electron oxidation of the diphenolic intermediate yield the final ortho‐quinone product, and one molecule of water. During the two‐electron oxidation step, the PPO copper center is reduced to its deoxy‐form, closing the catalytic monophenolase cycle. [45] Diphenolase activity (red): C) The diphenolic substrate is oxidized to the corresponding quinone by the dicopper center, which transitions from the oxy‐ to the met‐form. D) The met‐form accepts diphenolic substrates and converts them to the corresponding quinones. E) Similarly to the monophenolase activity, the PPO copper center is reduced to its deoxy‐form during substrate oxidation, thereby closing the catalytic diphenolase cycle. [2]
