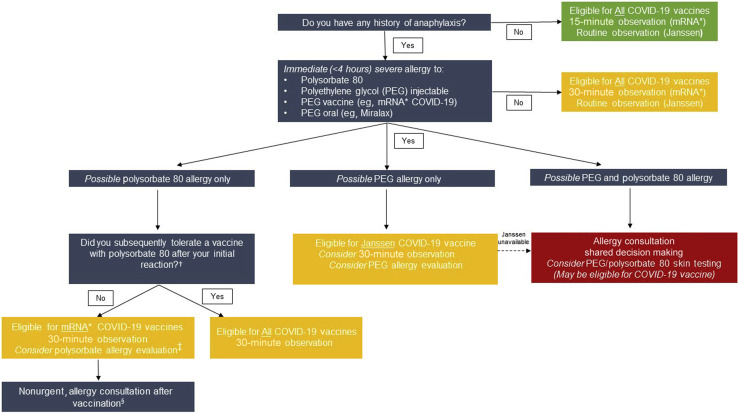
Figure 2.
Risk stratification pathways for COVID-19 vaccination in patients with possible PEG or polysorbate allergy. The primary role of the allergist is to enable patients to safely receive the first vaccine available to them. This may require allergist evaluation for PEG and/or polysorbate allergy depending on vaccine availability. Individuals with any history of anaphylaxis, per CDC guidance, would be monitored for 30 minutes after mRNA COVID-19 vaccination. Individuals without a PEG or polysorbate allergy are eligible to receive all COVID-19 vaccines and observation time would depend on which vaccine was being given and whether there was a previous history of anaphylaxis. Individuals with a polysorbate 80 only allergy would be further assessed by asking “Did you tolerate a polysorbate 80 vaccine after your initial reaction” to a polysorbate 80 injectable or vaccine. Individuals with PEG-only allergy are eligible to receive Janssen COVID-19 vaccine without allergy evaluation. ∗mRNA COVID-19 vaccines from Pfizer-BioNTech and Moderna. †See Table I. ‡Consider allergy evaluation of polysorbate allergy history if patient preference is for the Janssen vaccine and it is available. §Polysorbate allergy evaluation may be useful in guiding the future use of injectables and vaccines with polysorbate.