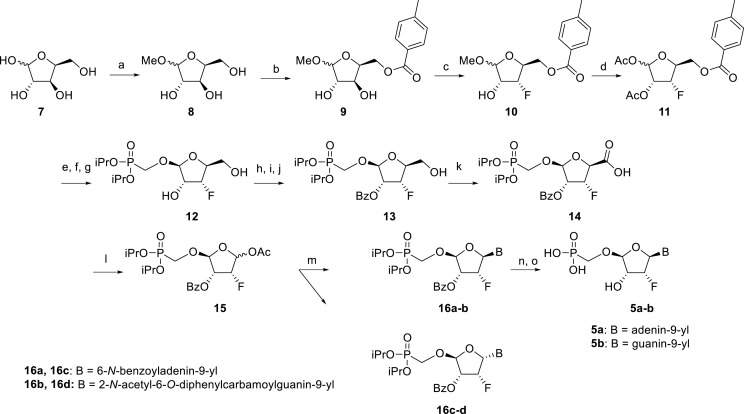
Scheme 1.
Synthesis of 2′-fluorotetradialdose nucleoside phosphonates. Reagents and conditions: (a) HCl, MeOH, rt, 16 h, quant.; (b) p-Toluoyl chloride, pyridine, 0 °C to rt, 16 h, 48%; (c) DAST, MeCN, 0 °C to rt, 16 h, 54%; (d) H2SO4, Ac2O, AcOH, 0 °C to rt, 2 h, 84%; (e) hexamethyldisilazane, (iPrO)2P(O)CH2OH, saccharine, 100 °C, 8 h; (f) SnCl4, MeCN, 55 °C, 1 h; (g) MeNH2, EtOH, rt, 16 h, 85%; (h) DMTrCl, pyridine, rt, 16 h; (i) BzCl, DMAP, pyridine, rt, 8 h; (j) TFA, DCM, rt, 10 min, 74%; (k) PhI(OAc)2, TEMPO, MeCN, rt, 16 h, 90%; (l) Pb(OAc)4, THF, rt, 16 h, 60%; (m) SnCl4, DCE, BSA, N6-benzoyladenine, rt, 30 min 43%; (n) TMSBr, pyridine, rt, 8 h; (o) MeNH2, EtOH, rt, 73%.