Table Ia.
1H NMR data of compounds 10–15 and 19–21.k
| Compound | Solvent | H-1 | H-2 | H-3 | H-4 | H-5a; H-5b |
|---|---|---|---|---|---|---|
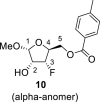 |
DMSO a | 4.86 d 1,2 = 4.7 |
4.045 bdq 2,1 = 4.7 2,F = 26.5 2, OH = 7.5 2,3 = 5.5 |
4.91 ddd 3,F = 56.2 3,2 = 5.5 3,4 = 1.4 |
4.44 dtd 4,F = 26.6 4,5b = 4.4 4,5a = 3.9 4,3 = 1.4 |
4.38 dd 5a, 5b = 11.8; 5a,4 = 3.9 4.35 dd 5b, 5a = 11.8; 5b,4 = 4.4 |
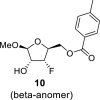 |
DMSO b | 4.77 t 1,2 = 2.0 1,F = 2.0 |
4.06 um | 5.08 dt 3,F = 53.7 3,2 = 4.5 3,4 = 4.5 |
4.42 m | 4.44 m 4.30 m |
 alpha + beta anomer (56:44) alpha + beta anomer (56:44) |
DMSO c | 6.09 d 1,2 = 2.6 1,F = 1.4 |
5.36 ddd 2,F = 11.0 2,3 = 4.8 2,1 = 2.6 |
5.52 ddd 3,F = 52.0 3,2 = 4.8 3,4 = 3.8 |
4.65 dq 4,F = 20.9 4,5b = 4.2 4,3 = 3.8 4,5a = 3.6 |
4.56 dd 5a, 5b = 12.2; 5a,4 = 3.6 4.39 dd 5b, 5a = 12.2; 5b,4 = 4.2 |
| 6.39 d 1,2 = 4.8 |
5.20 dt 2,F = 25.1 2,3 = 5.2 2,1 = 4.8 |
5.37 ddd 3,F = 55.0 3,2 = 5.2 3,4 = 1.3 |
4.76 dtd 4,F = 26.7 4,5b = 4.2 4,5a = 3.7 4,3 = 1.3 |
4.44 dd 5a, 5b = 12.0; 5a,4 = 3.7 4.42 dd 5b, 5a = 12.0; 5b,4 = 4.2 |
||
 |
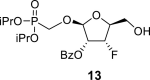
DMSO d | 4.905 dd 1,2 = 2.6 |
4.03 dddd 2,1 = 2.6 2,3 = 4.4 2, OH = 5.7 2,F = 11.7 |
4.86 dt 3,2 = 4.4 3.F = 53.9 3,4 = 3.8 |
4.08 dtd 4,3 = 3.8 4,F = 20.9 4,5a = 5.5 4,5b = 5.5 |
3.45 m (2H) |
 |
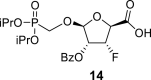
DMSO e | 5.40 dd 1,2 = 3.1 1,F = 1.4 |
5.31 ddd 2,1 = 3.1 2,3 = 4.9 2,F = 11.7 |
5.30 ddd 3,2 = 4.9 3.F = 53.6 3,4 = 3.1 |
4.29 dtd 4,3 = 3.1 4,F = 22.6 4,5a = 5.4 4,5b = 5.0 |
3.57 m (2H) |
 |
DMSO f | 5.47 dd 1,2 = 2.4 1,F = 1.3 |
5.34 ddd 2,1 = 2.4 2,3 = 4.7 2,F = 9.4 |
5.53 ddd 3,2 = 4.7 3.F = 52.6 3,4 = 3.4 |
4.77 dd 4,3 = 3.4 4,F = 23.2 |
– |
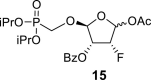 beta and alpha epimers (83:17) beta and alpha epimers (83:17) |
DMSO g data for major β-epimer only |
5.65 d 1,2 = 3.1 |
5.38 ddd 2,1 = 3.1 2,3 = 4.4 2,F = 17.0 |
5.44 ddd 3,2 = 4.4 3.F = 50.5 3,4 = 1.2 |
6.31 dd 4,3 = 1.2 4,F = 10.8 |
– |
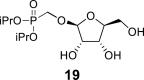 |
DMSO h | 4.80 s 1,2 = 0 |
3.74 bd 2,1 = 0 2,3 = 4.6 |
3.87 dd 3,2 = 4.6 3,4 = 7.2 |
3.78 ddd 4,3 = 7.2 4,5a = 3.2 4,5b = 6.1 |
3.54 dd 5a, 5b = 11.8; 5a,4 = 3.2 3.34 dd 5b, 5a = 11.8; 5b,4 = 4.4 |
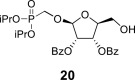 |
DMSO i | 5.38 d 1,2 = 1.5 |
5.51 dd 2,1 = 1.5 2,3 = 5.0 |
5.54 dd 3,2 = 5.0 3,4 = 5.8 |
4.42 ddd 4,3 = 5.8 4,5a = 4.6 4,5b = 5.1 |
3.68 ddd 5a, 5b = 12.0; 5a,4 = 4.6 5a, OH = 5.8 3.61 ddd 5b, 5a = 12.0; 5b,4 = 5.1 5b, OH = 6.0 |
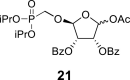 beta and alpha epimers (76:24) beta and alpha epimers (76:24) |
DMSO j | 5.65 d 1,2 = 2.1 |
5.60 dd 2,1 = 2.1 2,3 = 5.1 |
5.67 dd 3,2 = 5.1 3,4 = 2.4 |
6.46 d 4,3 = 2.4 |
– |
| 5.64 d 1,2 = 0.9 |
5.59 dd 2,1 = 0.9 2,3 = 5.1 |
5.67 dd 3,2 = 5.1 3,4 = 4.9 |
6.60 d 4,3 = 4.9 |
– |
1-OMe: 3.33 s; 2-OH: 5.12 br; 5-OTol: 7.84 m (2x o-ArH), 7.35 m (2x m-ArH), 2.385 s (CH3).
1-OMe: 3.22 s; 2-OH: 5.70 br; 5-OTol: 7.88 m (2x o-ArH), 7.35 m (2x m-ArH), 2.38 s (CH3).
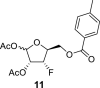
α-anomer: 1-OAc: 1.88 s; 2-OAc: 2.13 s; 5-OTol: 7.92 m (2x o-ArH), 7.36 m (2x m-ArH), 2.39 s (CH3); β-anomer: 1-OAc: 2.07 s; 2-OAc: 2.12 s; 5-OTol: 7.87 m (2x o-ArH), 7.34 m (2x m-ArH), 2.39 s (CH3).
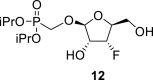
2-OH: 5.66 bd, J = 5.7 Hz; 5-OH: 4.96 bt, J = 5.5 Hz; O–CH2–P = O(OiPr)2: 3.84 dd, J = 13.8, 9.0 Hz and 3.73 dd, J = 13.8, 9.1 Hz (P–CH2–O), 4.59 m, 2H, 1.235 d, J = 6.2 Hz, 1.237 d, J = 6.2 Hz, 1.244 d, J = 6.2 Hz and 1.246 d, J = 6.2 Hz (2x OiPr).
2-OBz: 8.01 m (2x o-ArH), 7.57 m (2x m-ArH), 7.71 m (p-ArH); 5-OH: 5.14 bt, J = 5.6 Hz; O–CH2–P = O(OiPr)2: 3.95 dd, J = 13.9, 9.0 Hz and 3.87 dd, J = 13.9, 8.9 Hz (P–CH2–O), 4.69 m, 2H, 1.213 d, 3H, J = 6.2 Hz, 1.218 d, 3H, J = 6.2 Hz, 1.223 d, 3H, J = 6.2 Hz and 1.231 d, 3H, J = 6.2 Hz (2x OiPr).
2-OBz: 8.00 m (2x o-ArH), 7.57 m (2x m-ArH), 7.71 m (p-ArH); O–CH2–P = O(OiPr)2: 4.06 dd, J = 13.8, 8.2 Hz and 3.92 dd, J = 13.8, 9.4 Hz (P–CH2–O), 4.59 m, 2H, 1.210 d, 6H, J = 6.2 Hz, 1.221 d, 3H, J = 6.2 Hz, 1.225 d, 3H, J = 6.2 Hz (2x OiPr).
2-OBz: 8.02 m (2x o-ArH), 7.58 m (2x m-ArH), 7.73 m (p-ArH); 4-OAc: 2.10 s; O–CH2–P = O(OiPr)2: 3.92 m, 2H (P–CH2–O), 4.60 m, 2H, 1.205 d, 3H, J = 6.0 Hz, 1.213 d, 3H, J = 6.2 Hz, 1.216 d, 3H, J = 6.0 Hz and 1.231 d, 3H, J = 6.1 Hz (2x OiPr).
O–CH2–P = O(OiPr)2: 3.84 dd, J = 13.8, 8.9 Hz and 3.65 dd, J = 13.8, 8.7 Hz (P–CH2–O), 4.58 m, 2H, 1.245 d, 3H, J = 6.2 Hz, 1.242 d, 3H, J = 6.2 Hz and 1.234 d, 6H, J = 6.6 Hz (2x OiPr).
2-OBz: 7.87 m (2x o-ArH), 7.47 m (2x m-ArH), 7.65 m (p-ArH); 3-OBz: 7.85 m (2x o-ArH), 7.44 m (2x m-ArH), 7.62 m (p-ArH); 5-OH: 5.08 t, J = 5.8 and 6.0 Hz; O–CH2–P = O(OiPr)2: 4.00 dd, J = 13.8, 9.0 Hz and 3.87 dd, J = 13.8, 8.9 Hz (P–CH2–O), 4.64 m, 2H, 1.260 d, 6H, J = 6.2 Hz, 1.264 d, 3H, J = 6.2 Hz and 1.268 d, 3H, J = 6.2 Hz (2x OiPr).
Major epimer: 2-OBz: 7.88 m (2x o-ArH), 7.465 m (2x m-ArH), 7.655 m (p-ArH); 3-OBz: 7.88 m (2x o-ArH), 7.465 m (2x m-ArH), 7.655 m (p-ArH); O–CH2–P = O(OiPr)2: 3.95 dd, J = 13.7, 9.0 Hz and 3.92 dd, J = 13.7, 9.3 Hz (P–CH2–O), 4.64 m, 2H, 1.250 d, 3H, J = 6.2 Hz, 1.260 d, 3H, J = 6.2 Hz, 1.266 d, 3H, J = 6.2 Hz and 1.270 d, 3H, J = 6.2 Hz (2x OiPr); 4-OAc: 2.13 s; minor epimer: 2-OBz: 8.005 m (2x o-ArH), 7.565 m (2x m-ArH), 7.705 m (p-ArH); 3-OBz: 7.76 m (2x o-ArH), 7.42 m (2x m-ArH), 7.62 m (p-ArH); O–CH2–P = O(OiPr)2: 3.99 dd, J = 13.7, 9.0 Hz and 3.96 dd, J = 13.7, 8.7 Hz (P–CH2–O), 4.64 m, 2H, 1.252 d, 6H, J = 6.2 Hz, 1.273 d, 3H, J = 6.2 Hz and 1.277 d, 3H, J = 6.2 Hz (2x OiPr).
Coupling constants are written in italics in a shortened form (e.g. instead J(1‘,2‘) = 8.6 Hz we type simply 1,2 = 8.6).
