Table Ib.
13C,31P and19F NMR data of compounds 10–15 and 19–21k
| Compound | Solvent | C-1 | C-2 | C-3 | C-4 | C-5 | 31P | 19F |
|---|---|---|---|---|---|---|---|---|
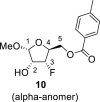 |
DMSO a | 102.64 | 71.74 2,F = 16.4 |
90.88 3,F = 185.2 |
80.09 4,F = 24.8 |
64.09 5,F = 10.3 |
– | −189.10 |
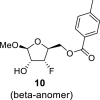 |
DMSO b | 108.19 1,F = 3.2 |
73.35 2,F = 15.0 |
92.11 3,F = 186.6 |
78.47 4,F = 25.0 |
64.03 5,F = 5.9 |
– | −205.56 |
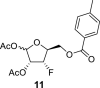 beta and alpha anomers (56:44) beta and alpha anomers (56:44) |
DMSO c β-epimer |
98.20 1,F = 1.9 |
74.69 2,F = 14.2 |
89.68 3,F = 189.9 |
81.91 4,F = 24.7 |
63.20 5,F = 6.8 |
– | −203.10 |
| α-epimer | 93.30 | 71.00 2,F = 14.7 |
88.84 3,F = 188.1 |
81.09 4,F = 24.8 |
63.63 5,F = 10.1 |
– | −190.71 | |
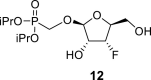 |
DMSO d | 107.71 1,P = 12.0 1,F = 2.4 |
73.56 2,F = 15.3 |
82.48 3,F = 185.8 |
82.32 4,F = 22.2 |
61.78 5,F = 6.3 |
20.56 | −203.93 |
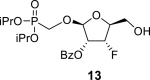 |
DMSO e | 105.30 1,P = 12.2 1,F = 1.4 |
75.56 2,F = 14.2 |
90.81 3,F = 188.1 |
83.40 4,F = 21.9 |
61.29 5,F = 7.3 |
19.95 | −201.10 |
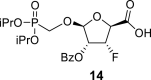 |
DMSO f | 105.47 1,P = 12.1 |
74.82 2,F = 13.8 |
92.21 3,F = 193.1 |
80.56 4,F = 23.4 |
170.81 5,F = 9.2 |
19.92 | −199.95 |
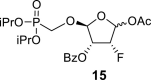 beta and alpha epimers (83:17) beta and alpha epimers (83:17) |
DMSO g data for major β-epimer only |
107.10 1,P = 13.4 |
75.26 2,F = 14.3 |
91.96 3,F = 188.8 |
98.26 4,F = 33.1 |
– | 18.98 | −206.24 |
 |
DMSO h | 107.73 1,P = 11.6 |
74.40 | 70.73 | 84.03 | 62.80 | 21.08 | – |
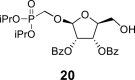 |
DMSO i | 105.23 1,P = 12.2 |
74.87 | 72.27 | 82.20 | 61.98 | 20.12 | – |
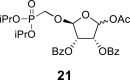 beta and alpha epimers (76:24) |
DMSO j β-epimer |
106.98 1,P = 13.1 |
74.63 | 75.29 | 98.82 | – | 18.03 | – |
| α-epimer | 105.53 1,P = 12.3 |
72.28 | 70.16 | 93.14 | – | 19.19 | – |
Substituents.
1-OMe: 55.11; 2-OTol: 165.61 (C O), 126.77 (i-ArC), 129.43 (2x o-ArC), 129.61 (2x m-ArC), 144.11 (p-AQrC), 21.38 (CH3).
1-OMe: 55.16; 2-OTol: 165.65 (C O), 126.92 (i-ArC), 129.46 (2x o-ArC), 129.53 (2x m-ArC), 144.03 (p-ArC), 21.37 (CH3).
α-anomer: 1-OAc: 169.19 (C O), 20.74 (CH3); 2-OAc: 169.53 (C O), 20.48 (CH3); 2-OTol: 165.42 (C O), 126.71 (i-ArC), 129.53 (2x o-ArC), 129.58 (2x m-ArC), 144.20 (p-ArC), 21.35 (CH3); β-anomer: 1-OAc: 169.77 (C O), 21.06 (CH3); 2-OAc: 169.73 (C O), 20.40 (CH3); 2-OTol: 165.50 (C O), 126.59 (i-ArC), 129.48 (2x o-ArC), 129.56 (2x m-ArC), 144.20 (p-ArC), 21.34 (CH3).
O–CH2–P = O(OiPr)2: 61.12 d, J = 166.9 Hz (P–CH2–O), 70.70 d, J = 5.0 Hz and 70.57 d, J = 5.0 Hz (2x O–CH<), 23.93 d, J = 4.6 Hz (2x CH3), and 24.06 d, J = 3.4 Hz (2x CH3).
2-OBz: 164.92 (C O), 128.74 (i-ArC), 129.67 (2x o-ArC), 129.22 (2x m-ArC), 134.29 (p-ArC), O–CH2–P = O(OiPr)2: 61.46 d, J = 166.3 Hz (P–CH2–O), 70.81 d, J = 6.3 Hz (2x O–CH<), 23.88 d, J = 4.6 Hz (CH3), 23.87 d, J = 4.6 Hz (CH3), 24.03 d, J = 3.7 Hz (CH3) and 24.03 d, J = 3.7 Hz (CH3).
2-OBz: 164.82 (C O), 128.61 (i-ArC), 129.68 (2x o-ArC), 129.19 (2x m-ArC), 134.30 (p-ArC), O–CH2–P = O(OiPr)2: 60.85 d, J = 165.6 Hz (P–CH2–O), 70.77 d, J = 6.2 Hz and 70.80 d, J = 6.2 Hz (2x O–CH<), 23.82 d, J = 4.7 Hz (CH3), 23.86 d, J = 4.7 Hz (CH3), 23.97 d, J = 3.6 Hz (CH3) and 24.00 d, J = 3.8 Hz (CH3).
2-OBz: 164.86 (C O), 128.41 (i-ArC), 129.81 (2x o-ArC), 129.32 (2x m-ArC), 134.54 (p-ArC), 4-OAc: 169.17 (C O), 21.03 (CH3); O–CH2–P = O(OiPr)2: 62.32 d, J = 166.0 Hz (P–CH2–O), 70.95 d, J = 6.2 Hz and 70.93 d, J = 6.1 Hz (2x O–CH<), 23.87 d, J = 4.4 Hz (2x CH3), 24.03 d, J = 3.5 Hz (2x CH3).
O–CH2–P = O(OiPr)2: 60.47 d, J = 166.9 Hz (P–CH2–O), 70.46 d, J = 6.0 Hz and 70.42 d, J = 6.0 Hz (2x O–CH<), 24.02 d, J = 4.5 Hz (2x CH3), 23.89 d, J = 4.8 Hz (2x CH3).
2-OBz: 164.73 (C O), 128.88 (i-ArC), 129.43 (2x o-ArC), 129.01 (2x m-ArC), 134.09 (p-ArC), 3-OBz: 165.06 (C O), 128.73 (i-ArC), 129.37 (2x o-ArC), 128.92 (2x m-ArC), 133.94 (p-ArC); O–CH2–P = O(OiPr)2: 61.07 d, J = 166.6 Hz (P–CH2–O), 70.71 d, J = 6.3 Hz (2x O–CH<), 23.86 d, J = 4.5 Hz (2x CH3), 24.00 d, J = 3.7 Hz (2x CH3).
Major β-epimer: 2-OBz: 164.67 (C O), 128.42 (i-ArC), 129.56 (2x o-ArC), 129.03 (2x m-ArC), 134.25 (p-ArC), 3-OBz: 164.62 (C O), 128.41 (i-ArC), 129.50 (2x o-ArC), 129.00 (2x m-ArC), 134.21 (p-ArC); O–CH2–P = O(OiPr)2: 61.88 d, J = 166.0 Hz (P–CH2–O), 70.83 d, J = 6.2 Hz and 70.77 d, J = 6.2 Hz (2x O–CH<), 23.81 d, 2C, J = 4.6 Hz (2x CH3), 23.96 d, J = 3.5 Hz and 23.97 d, J = 3.7 Hz (2x CH3); 4-OAc: 169.39 (C O), 20.95 (CH3); minor α-epimer: 2-OBz: 164.70 (C O), 128.83 (i-ArC), 129.54 (2x o-ArC), 129.14 (2x m-ArC), 134.23 (p-ArC), 3-OBz: 164.46 (C O), 128.44 (i-ArC), 129.28 (2x o-ArC), 129.03 (2x m-ArC), 134.15 (p-ArC); O–CH2–P = O(OiPr)2: 61.82 d, J = 166.1 Hz (P–CH2–O), 70.83 d, J = 6.2 Hz and 70.77 d, J = 6.2 Hz (2x O–CH<), 23.98 d, J = 3.7 Hz (2x CH3), 23.86 d, J = 4.6 Hz (CH3) and 23.84 d, J = 4.6 Hz (CH3); 4-OAc: 169.27 (C O), 20.85 (CH3).
Coupling constants are written in italics in a shortened form (e.g. instead J(C2,F) = 16.4 Hz we type simply 2,F = 16.4).
