Table IIa.
1H NMR data of compounds 5, 6, 16 and 22–27x
| Compound | Solvent | H-1‘ | H-2‘ | H-3‘ | H-4‘ | Base |
|---|---|---|---|---|---|---|
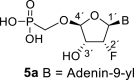 |
D2O a | 6.44 dd 1,2 = 4.3 1,F = 14.6 |
5.71 dt 2,F = 50.8 2,1 = 4.3 2,3 = 4.4 |
4.59 ddd 3,2 = 4.4 3,F = 7.1 3,4 = 2.0 |
5.33 dd 4,3 = 2.0 4,F = 1.7 |
H-2: 8.15 s H-8: 8.33 s |
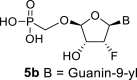 |
D2O b | 6.31 dd 1,2 = 4.2 1,F = 15.4 |
5.72 dt 2,F = 51.1 2,1 = 4.2 2,3 = 4.4 |
4.62 ddd 3,2 = 4.4 3,F = 8.1 3,4 = 2.3 |
5.32 dd 4,3 = 2.3 4,F = 1.6 |
H8: 8.06 s |
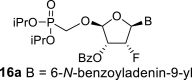 |
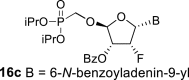
DMSO c | 6.74 dd 1,2 = 3.6 1,F = 17.0 |
6.35 ddd 2,1 = 3.6 2,F = 49.8 2,3 = 4.9 |
5.91 ddd 3,2 = 4.9 3,F = 9.2 3,4 = 2.5 |
5.71 dd 4,3 = 2.5 4,F = 1.0 |
H-2: 8.80 s H-8: 8.65 s |
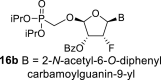 |
DMSO d | 6.59 dd 1,2 = 3.2 1,F = 17.6 |
6.31 ddd 2,1 = 3.2 2,F = 49.9 2,3 = 5.0 |
6.02 ddd 3,2 = 5.0 3,F = 10.3 3,4 = 2.7 |
5.70 dd 4,3 = 2.7 4,F = 0.6 |
H-8: 8.59 s |
 |
DMSO e | 6.995 dd 1,2 = 3.7 1,F = 16.8 |
5.79 ddd 2,1 = 3.7 2,F = 52.2 2,3 = 4.5 |
5.72 ddd 3,2 = 4.5 3,F = 15.3 3,4 = 3.5 |
5.95 dd 4,3 = 3.5 4,F = 1.3 |
H-2: 8.81 s H-8: 8.60 d 8,F = 2.7 |
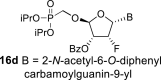 |
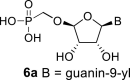
DMSO f | 6.82 dd 1,2 = 3.8 1,F = 16.2 |
5.74 ddd 2,1 = 3.8 2,F = 52.1 2,3 = 4.7 |
5.67 ddd 3,2 = 4.7 3,F = 16.1 3,4 = 3.9 |
6.08 dd 4,3 = 3.9 4,F = 1.2 |
H-8: 8.55 d 8,F = 2.5 |
 |
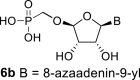
D2O g | 6.02 d 1,2 = 6.5 |
4.97 ddd 2,1 = 6.5 2,3 = 4.4 2,4 = 0.6 |
4.37 dt 3,2 = 4.4 3,4 = 0.6 3,P = 0.6 |
5.21 t 4,3 = 0.6 4,2 = 0.6 |
H-8: 8.09 s |
 |
D2O h | 6.42 d 1,2 = 5.8 |
5.55 dd 2,1 = 5.8 2,3 = 4.6 |
4.52 dd 3,2 = 4.6 3,4 = 0.7 |
5.31 d 4,3 = 0.7 |
H-2: 8.34 s |
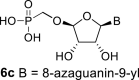 |
D2O i | 6.20 d 1,2 = 6.1 |
5.39 dd 2,1 = 6.1 2,3 = 4.6 |
4.46 dd 3,2 = 4.6 3,4 = 0.8 |
5.27 d 4,3 = 0.8 |
– |
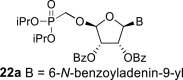 |
DMSO j | 6.87 d 1,2 = 5.7 |
6.51 dd 2,1 = 5.7 2,3 = 5.0 |
5.96 dd 3,2 = 5.0 3,4 = 1.1 |
5.70 d 4,3 = 1.1 |
H-2: 8.78 s H-8: 8.71 s |
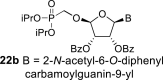 |
DMSO k | 6.71 d 1,2 = 5.7 |
6.46 dd 2,1 = 5.7 2,3 = 5.0 |
5.94 dd 3,2 = 5.0 3,4 = 1.2 |
5.68 d 4,3 = 1.2 |
H-8: 8.645 s |
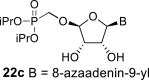 |
DMSO l | 6.245 d 1,2 = 6.4 |
5.22 td 2,1 = 6.4 2,3 = 4.4 2, OH = 6.7 |
4.15 td 3,2 = 4.4 3,4 = 1.1 3, OH = 4.5 |
5.68 d 4,3 = 1.1 |
H-2: 8.33 s NH2: 8.53 br and 8.18 br |
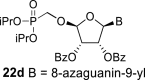 |
DMSO m | 6.59 d 1,2 = 4.7 |
6.52 t 2,1 = 4.7 2,3 = 4.8 |
5.93 dd 3,2 = 4.7 3,4 = 2.1 |
5.72 d 4,3 = 2.1 |
CO–NH: 11.15 bs |
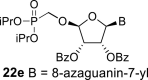 |
DMSO n | 6,94 d 1,2 = 4.0 |
6.56 dd 2,1 = 4.0 2,3 = 5.1 |
5.95 dd 3,2 = 5.1 3,4 = 2.3 |
5.765 d 4,3 = 2.3 |
CO–NH: 11.47 bs NH2: 6.60 br |
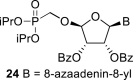 |
DMSO o | 6.95 d 1,2 = 4.2 |
6.70 q 2,1 = 4.2 2,3 = 5.1 |
6.03 dd 3,2 = 5.1 3,4 = 2.2 |
5.76 d 4,3 = 2.2 |
H-2: 8.36 s NH2: 8.67 br and 8.26 br |
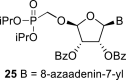 |
DMSO p | 7.14 d 1,2 = 6.4 |
6.18 t 2,1 = 6.4 2,3 = 6.0 |
5.73 dd 3,2 = 6.0 3,4 = 1.3 |
6.10 d 4,3 = 1.3 |
H-2: 8.295 s NH2: 8.37 br and 8.15 br |
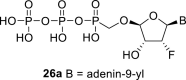 |
D2O q | 6.52 dd 1,2 = 4.3 1,F = 14.5 |
5.78 dt 2,F = 50.8 2,1 = 4.3 2,3 = 4.5 |
4.65 ddd 3,2 = 4.5 3,4 = 1.8 3,F = 7.0 |
5.40 t 4,3 = 1.8 4,F = 1.8 |
H-2: 8.24 s H-8: 8.42 s |
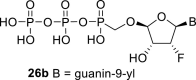 |
D2O r | 6.32 dd 1,2 = 4.5 1,F = 15.1 |
5.77 dt 2,F = 51.0 2,1 = 4.5 2,3 = 4.5 |
4.66 ddd 3,2 = 4.5 3,4 = 1.8 3,F = 6.7 |
5.36 t 4,3 = 1.8 4,F = 1.9 |
H-8: 8.05 s |
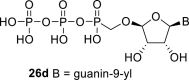 |
D2O s | 6.04 d 1,2 = 6.7 |
5.01 ddd 2,1 = 6.7 2,3 = 4.5 2,4 = 0.5 |
4.42 dt 3,2 = 4.5 3,4 = 0.6 3,P = 0.6 |
5.27 t 4,3 = 0.6 4,2 = 0.5 |
H-8: 8.09 s |
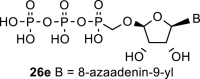 |
D2O t | 6.43 d 1,2 = 6.2 |
5.56 dd 2,1 = 6.2 2,3 = 4.6 |
4.555 dd 3,2 = 6.2 3,4 = 0.9 |
5.395 d 4,3 = 0.9 |
H-2: 8.37 s |
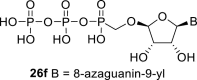 |
D2O u | 6.235 d 1,2 = 6.6 |
5.43 dd 2,1 = 6.6 2,3 = 4.6 |
4.49 bd 3,2 = 4.6 3,4 < 1.0 |
5.34 bs 4,3 < 1.0 |
– |
 Mixture of diastereomers 53:47 |
DMSO v Major |
6.39 dd 1,2 = 5.0 1,F = 14.7 |
5.81 dt 2,F = 51.3 2,1 = 5.0 2,3 = 4.5 |
4.37 qd 3,2 = 4.5 3,4 = 1.7 3,F = 5.0 3, OH = 5.0 |
5.16 t 4,3 = 1.7 4,F = 1.7 |
H-2: 8.17 s H-8: 8.25 s |
| Minor | 6.38 dd 1,2 = 5.0 1,F = 14.7 |
5.78 dt 2,F = 51.3 2,1 = 5.0 2,3 = 4.5 |
4.355 qd 3,2 = 4.5 3,4 = 1.7 3,F = 5.0 3, OH = 5.0 |
5.02 t 4,3 = 1.7 4,F = 1.7 |
H-2: 8.17 s H-8: 8.21 s |
|
 Mixture of diastereomers 57:43 |
DMSO w Major |
6.175 dd 1,2 = 5.4 1,F = 14.3 |
5.605 dt 2,F = 51.6 2,1 = 5.4 2,3 = 4.3 |
4.27 qd 3,2 = 4.3 3,4 = 1.7 3,F = 5.0 3, OH = 5.1 |
5.11 t 4,3 = 1.7 4,F = 1.6 |
H-8: 7.79 s |
| minor | 6.16 dd 1,2 = 5.6 1,F = 14.2 |
5.58 dt 2,F = 51.7 2,1 = 5.6 2,3 = 4.3 |
4.25 qd 3,2 = 4.3 3,4 = 1.6 3,F = 5.0 3, OH = 5.1 |
4.96 t 4,3 = 1.6 4,F = 1.5 |
H-8: 7.77 s |
Substituents.
O–CH2–P = O(OH)2: 3.81 dd, J = 12.9, 8.9 Hz and 3.62 dd, J = 12.9, 9.7 Hz.
O–CH2–P = O(OH)2: 3.76 m and 3.53 m.
3-OBz: 8.10 m (2x o-ArH), 7.62 m (2x m-ArH), 7.465 m (p-ArH); O–CH2–P = O(OiPr)2: 3.97 dd, J = 13.9; 9.1 Hz and 3.92 dd, J = 13.9; 9.1 Hz (P–CH2–O), 4.54 m (2x O–CH<), 1.204 d, J = 6.2 Hz, 1.207 d, J = 6.2 Hz, 1.233 d, J = 6.2 Hz, 1.238 d, J = 6.2 Hz (2x OiPr); NHBz: 11.30 br (NH), 8.05 m (2x o-ArH), 7.56 m (2x m-ArH), 7.56 m (p-ArH).
NHAc: 10.82 s (NH), 2.18 s (CH3); 3-OBz: 8.07 m (2x o-ArH), 7.61 m (2x m-ArH), 7.75 m (p-ArH); O–CH2–P = O(OiPr)2: 3.95 d, J = 9.5 Hz (P–CH2–O), 4.57 m (2x O–CH<), 1.121 d, J = 6.2 Hz, 1.156 d, J = 6.2 Hz, 1.160 d, J = 6.2 Hz, 1.186 d, J = 6.2 Hz (2x OiPr); O–CO–N(C6H5)2: 7.51 m (4x o-ArH), 7.45 m (4x m-ArH), 7.33 (2x p-ArH).
3-OBz: 8.05 m (2x o-ArH), 7.60 m (2x m-ArH), 7.735 m (p-ArH); O–CH2–P = O(OiPr)2: 4.08 dd, J = 14.0; 9.2 Hz and 4.02 dd, J = 14.0; 9.4 Hz (P–CH2–O), 4.64 m (2x O–CH<), 1.234 d, J = 6.2 Hz, 1.240 d, 6H, J = 6.2 Hz and 1.254 d, J = 6.2 Hz (2x OiPr); NHBz: 11.29 bs (NH), 8.055 m (2x o-ArH), 7.56 m (2x m-ArH), 7.65 (p-ArH).
3-OBz: 8.00 m (2x o-ArH), 7.57 m (2x m-ArH), 7.72 m (p-ArH); O–CH2–P = O(OiPr)2: 4.05 dd, J = 13.9; 9.0 Hz and 4.035 dd, J = 13.9; 9.1 Hz (P–CH2–O), 4.63 m (2x O–CH<), 1.214 d, J = 6.2 Hz, 1.225 d, J = 6.2 Hz, 1.226 d, J = 6.2 Hz and 1.239 d, J = 6.2 Hz (2x OiPr); NHAc: 10.76 s (NH), 2.23 s (CH3); O–CO–N(C6H5)2: 7.49 br m (4x o-ArH), 7.44 m (4x m-ArH), 7.32 (2x p-ArH).
O–CH2–P = O(OH)2: 3.75 dd, J = 12.8, 8.6 Hz and 3.50 dd, J = 12.8, 9.8 Hz.
O–CH2–P = O(OH)2: 3.62 dd, J = 13.4, 8.4 Hz and 3.50 dd, J = 13.4, 9.5 Hz.
O–CH2–P = O(OH)2: 3.67 dd, J = 13.1, 8.1 Hz and 3.46 dd, J = 13.1, 9.7 Hz.
3-OBz: 7.82 m (2x o-ArH), 7.43 m (2x m-ArH), 7.625 m (p-ArH); 3-OBz: 8.045 m (2x o-ArH), 7.57 m (2x m-ArH), 7.72 m (p-ArH); O–CH2–P = O(OiPr)2: 3.98 m (P–CH2–O), 4.65 m (2x O–CH<), 1.247 d, J = 6.2 Hz, 1.255 d, J = 6.2 Hz, 1.266 d, 6H, J = 6.4 Hz (2x OiPr); NHBz: 11.27 br (NH), 8.055 m (2x o-ArH), 7.555 m (2x m-ArH), 7.65 m (p-ArH).
2-OBz: 7.80 m (2x o-ArH), 7.41 m (2x m-ArH), 7.61 m (p-ArH); 3-OBz: 8.005 m (2x o-ArH), 7.56 m (2x m-ArH), 7.71 m (p-ArH); O–CH2–P = O(OiPr)2: 3.97 d, J = 9.4 Hz (P–CH2–O), 4.65 m (2x O–CH<), 1.236 d, J = 6.2 Hz, 1.256 d, J = 6.2 Hz, 1.263 d, J = 6.0 Hz and 1.273 d, J = 6.0 Hz (2x OiPr); NHAc: 10.76 s (NH), 2.215 s (CH3); O–CO–N(C6H5)2: 7.505 m (4x o-ArH), 7.44 m (4x m-ArH), 7.32 (2x p-ArH).
O–CH2–P = O(OH)2: 3.69 d, 2H, J = 8.8 Hz; 2-OH: 5.66 d, J = 6.7 Hz; 3-OH: 5.73 d, J = 4.5 Hz.
2-OBz: 7.97 m (2x o-ArH), 7.53 m (2x m-ArH), 7.70 m (p-ArH); 3-OBz: 7.85 m (2x o-ArH), 7.45 m (2x m-ArH), 7.64 m (p-ArH); O–CH2–P = O(OiPr)2: 3.88 d, 2H, J = 9.0 Hz (P–CH2–O), 4.58 m (2x O–CH<), 1.196 d, J = 6.2 Hz, 1.209 d, J = 6.2 Hz, 1.222 d, J = 6.2 Hz and 1.235 d, J = 6.2 Hz (2x OiPr).
2-OBz: 7.94 m (2x o-ArH), 7.51 m (2x m-ArH), 7.69 m (p-ArH); 3-OBz: 7.87 m (2x o-ArH), 7.46 m (2x m-ArH), 7.65 m (p-ArH); O–CH2–P = O(OiPr)2: 3.88 dd, J = 13.9; 9.4 Hz and 3.80 dd, J = 13.9; 8.5 Hz, (P–CH2–O), 4.56 m (2x O–CH<), 1.165 d, J = 6.2 Hz 1.196 d, J = 6.2 Hz, 1.202 d, J = 6.2 Hz and 1.208 d, J = 6.2 Hz (2x OiPr).
2-OBz: 7.86 m (2x o-ArH), 7.44 m (2x m-ArH), 7.645 m (p-ArH); 3-OBz: 8.00 m (2x o-ArH), 7.52 m (2x m-ArH), 7.70 m (p-ArH); O–CH2–P = O(OiPr)2: 3.88 dd, J = 13.9; 9.4 Hz and 3.78 dd, J = 13.9; 8.6 Hz, (P–CH2–O), 4.55 m (2x O–CH<), 1.141 d, J = 6.2 Hz, 1.185 d, J = 6.2 Hz, 1.187 d, J = 6.2 Hz and 1.202 d, J = 6.2 Hz (2x OiPr).
2-OBz: 7.24 m (2x o-ArH), 7.15 m (2x m-ArH), 7.46 m (p-ArH); 3-OBz: 8.00 m (2x o-ArH), 7.45 m (2x m-ArH), 7.64 m (p-ArH); O–CH2–P = O(OiPr)2: 4.14 dd, J = 14.0; 9.0 Hz and 4.12 dd, J = 14.0; 8.8 Hz, (P–CH2–O), 4.68 m (2x O–CH<), 1.282 d, J = 6.2 Hz, 1.285 d, J = 6.2 Hz and 1.290 d, 6H, J = 6.2 Hz (2x OiPr).
O–CH2–P( = O)(OH)–O–P( = O)(OH)–O–P( = O)(OH)2: 3.99 dd, J = 13.3; 8.5 Hz and 3.89 dd, J = 13.2; 10.1 Hz (P–CH2–O).
O–CH2–P( = O)(OH)–O–P( = O)(OH)–O–P( = O)(OH)2: 3.98 dd, J = 13.3; 8.4 Hz and 3.86 dd, J = 13.3; 10.3 Hz (P–CH2–O).
O–CH2–P( = O)(OH)–O–P( = O)(OH)–O–P( = O)(OH)2: 3.95 dd, J = 13.3; 8.2 Hz and 3.82 dd, J = 13.3; 10.3 Hz (P–CH2–O).
O–CH2–P( = O)(OH)–O–P( = O)(OH)–O–P( = O)(OH)2: 3.775 dd, J = 13.8; 7.7 Hz and 3.75 dd, J = 13.8; 9.1 Hz (P–CH2–O).
O–CH2–P( = O)(OH)–O–P( = O)(OH)–O–P( = O)(OH)2: 3.875 dd, J = 13.6; 7.4 Hz and 3.765 dd, J = 13.6; 9.9 Hz (P–CH2–O).
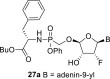
Major diastereomer: 3-OH: 6.165 d, J = 5.0 Hz; Bu-O-CH(CH2C6H5)–CO–NH–P( = O)(O–C6H5)–CH2–O: 0.81 t, 3H, J = 7.4 Hz, 1.18 m, 2H, 1.39 m, 2H, 3.92 m, 2H (Bu-O-); 4.05 m, 1H, 2.94 m, 1H and 2.735 m, 1H (O–CH–CH2); 7.38 br, 1H (NH); 7.00–7.30 m, 10H (10x ArH); 3.45 dd, 1H, J = 13.5; 8.8 Hz and 3.405 dd 1H, J = 13.5; 8.2 Hz (P–CH2–O); minor diastereomer: 3-OH: 6.155 d, J = 5.0 Hz; Bu-O-CH(CH2C6H5)–CO–NH–P( = O)(O–C6H5)–CH2–O: 0.785 t, 3H, J = 7.4 Hz, 1.18 m, 2H, 1.39 m, 2H, 3.90 m, 2H (Bu-O-); 4.07 m, 1H, 2.91 m, 1H and 2.72 m, 1H (O–CH–CH2); 7.395 br, 1H (NH); 7.00–7.30 m, 10H (10x ArH); 3.84 dd, 1H, J = 13.7; 8.8 Hz and 3.69 dd 1H, J = 13.7; 7.0 Hz (P–CH2–O).
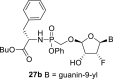
Major diastereomer: 3-OH: 6.13 d, J = 5.1 Hz; Bu-O-CH(CH2C6H5)–CO–NH–P( = O)(O–C6H5)–CH2–O: 0.805 t, 3H, J = 7.4 Hz, 1.195 m, 2H, 1.41 m, 2H, 3.93 m, 2H (Bu-O-); 4.07 m, 1H, 2.915 ddd, 1H, J = 13.6; 6.0; 2.0 Hz and 2.72 dd, 1H, J = 13.6; 8.5 Hz (O–CH–CH2); 7.38 br, 1H (NH); 7.025–7.275 m, 10H (10x ArH); 3.88 dd, 1H, J = 13.5; 9.1 Hz and 3.695 dd 1H, J = 13.5; 7.2 Hz (P–CH2–O); minor diastereomer: 3-OH: 6.12 d, J = 5.1 Hz; Bu-O-CH(CH2C6H5)–CO–NH–P( = O)(O–C6H5)–CH2–O: 0.805 t, 3H, J = 7.4 Hz, 1.195 m, 2H, 1.41 m, 2H, 3.905 m, 2H (Bu-O-); 4.07 m, 1H, 2.96 ddd, 1H, J = 13.5; 6.2; 1.5 Hz and 2.75 dd, 1H, J = 13.5; 9.1 Hz (O–CH–CH2); 7.395 br, 1H (NH); 7.025–7.275 m, 10H (10x ArH); 3.46 dd, 1H, J = 13.5; 8.3 Hz and 3.43 dd, 1H, J = 13.5; 8.9 Hz (P–CH2–O).
Coupling constants are written in italics in a shortened form (e.g. instead J(1‘,2‘) = 4.3 Hz we type simply 1,2 = 4.3).
