Figure 3. Bioengineered in vitro vascular models.
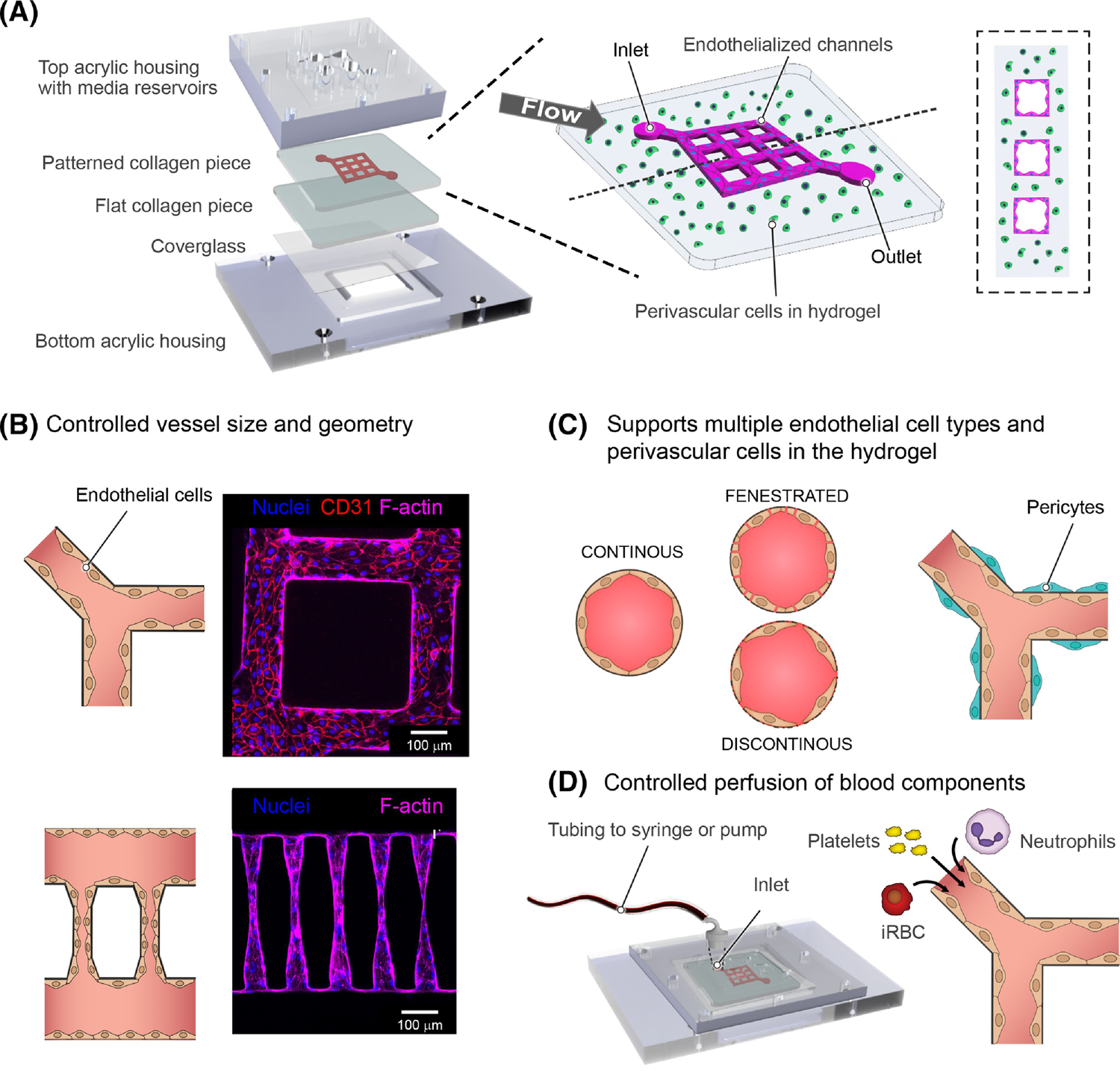
a. Rendering of device components (left), and schematic representation of 3D microvessels with a 3x3 grid microfluidic network (right). Bioengineered 3D microvessels are a versatile model. b. By combining fabrication methods like soft-lithography and photoablation control can be achieved over vessel size, diameter and network geometry. This is exemplified by immunofluorescences showing a portion of a 13X13 grid composed of a network of 100 μm diameter vessels (top), and vessels mimicking arteriole-capillary-venule transition with a diameter of 5–15 μm in the narrowest region (bottom). c. Engineered 3D microvessels are compatible with multiple endothelial cell types including continuous, fenestrated and sinusoidal endothelium, and support the growth of perivascular and parenchyma cells within the hydrogel. d. The use of a predetermined network provides refined control over microfluidic properties when connected to a flow pump. Perfusion of different blood components through the device can be independent, sequential or combined to understand the independent or synergistic contribution of parasite and host components in CM.
