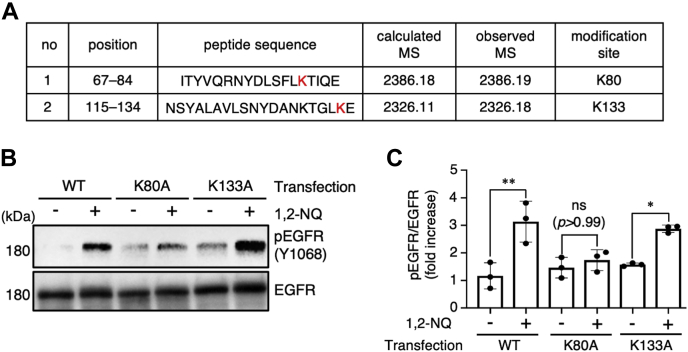
Figure 3.
1,2-NQ forms N-arylation with EGFR at Lys80 and induces its phosphorylation.A, identification of 1,2-NQ–binding sites in recombinant extracellular EGFR by ultra–high-performance liquid chromatography–tandem mass spectrometry analysis. Recombinant EGFR (5 μM) was incubated with 25 μM 1,2-NQ for 15 min at 25 °C in 50 mM ammonium bicarbonate. Trypsin-digested peptides were analyzed by ultra–high-performance liquid chromatography–tandem mass spectrometry. The mass data are shown in Fig. S4. B and C, human embryonic kidney 293T cells were transiently transfected with WT, K80A, or K133A human EGFR. Cells were incubated with serum-free medium for 18 h after 6 h of transfection and were then stimulated with 10 μM 1,2-NQ for 10 min. The relative level of phosphorylated EGFR was normalized to the level of total EGFR. Statistical analysis was carried out by one-way ANOVA with Bonferroni's multiple comparisons test. All data are expressed as the mean ± SEM values. n = 3, ∗p < 0.05, and ∗∗p < 0.01 versus control. 1,2-NQ, 1,2-naphthoquinone; EGFR, epidermal growth factor receptor; ns, not significant.