Abstract
Vascular cognitive impairment (VCI) is a term that encompasses a continuum of cognitive disorders with cerebrovascular pathology contribution, ranging from mild cognitive impairment to vascular dementia (VaD). VCI and VaD, thus, represent an interesting intersection between cardiovascular disease and neurodegenerative disorders such as Alzheimer’s disease (AD) and a rising area of research in recent years. Although VCI and VaD research has identified various causes and explanations for disease development, many aspects remain unclear, particularly sex differences in VCI (e.g., epidemiology), unlike those available for cardiovascular disease and AD. Despite limited information in the literature, several studies have observed an association of estrogen receptor (ER) polymorphisms and VaD. If further explored, this association could provide valuable insights for novel therapeutic approaches. This review aims to provide a brief epidemiological overview and subsequent discussion exploring concepts of brain aging and involvement of estrogen receptors in potential mechanisms of VCI/VaD pathogenesis and treatment development.
Keywords: Vascular cognitive impairment (VCI), Vascular dementia (VaD), Estrogen receptor (ER), Brain aging, Alzheimer’s disease (AD)
Introduction
Increase in lifespan of the aging population worldwide is creating a greater demand for cardiovascular and neurobiology of aging research, particularly since prevalence of various pathologies (e.g., stroke and dementia) increases exponentially with age [1, 2]. Vascular cognitive impairment (VCI) is a term which encompasses a continuum of cognitive deficits with cerebrovascular pathology contribution, ranging from mild cognitive impairment to vascular dementia (VaD) [3, 4], and it represents an interesting intersection between cardiovascular disease and Alzheimer’s disease (AD). There is an expanding body of evidence indicating a greater prevalence of cardiovascular disease [5, 6] and AD [7, 8] in postmenopausal women, as well as differences in pathogenesis and response to treatment [9–12]. However, one important aspect that remains elusive in the literature is information on sex differences in VCI. Several studies have reported associations of estrogen receptor (ER) polymorphisms with VaD [13–15]; however, this has not been extensively reviewed in terms of sex differences in VCI epidemiology, pathogenesis, and treatment. Particularly lacking is epidemiological data on sex differences in prevalence of VCI, which is possibly due to the complication of VCI being a spectrum of cognitive disorders with multiple etiologies as well as having varying definitions in the literature [1, 3, 4]. This review aims to provide a brief epidemiological overview and subsequent discussion exploring concepts of brain aging and involvement of estrogen receptors in potential mechanisms of VCI/VaD pathogenesis and treatment development. To mitigate the aforementioned complication of VCI definitions, a similar approach taken by van der Flier et al. [4] will be used here in which VCI is regarding the spectrum of cognitive deficits with vascular pathology, and both VaD and VCI will be used as they appear in the literature.
Epidemiology of vascular cognitive impairment and vascular dementia
Demographics and risk factors
Despite the major inconsistencies in the epidemiological data of VCI and VaD, with a disproportional leaning toward more data available for VaD, certain aspects of demographics and risk factors have been more definitive. Numerous studies have agreed on VaD being the second most common type of dementia, with majority of the cases in patients over age 70 [16, 17]. Studies conducted at the turn and early parts of the century reported the incidence of new VaD cases to be 6–12 cases per 1000 people over age 70 [18, 19] and the prevalence of VaD estimated to be 594,000 US cases in 2002 (71–79 years old 0.98%, 80–89 years old 4.09%, ≥ 90 years old 6.19%) [16, 20]. However, due to the lack of contemporary population-based studies and updates on these data, there is limited information on VaD prevalence changes as well as sex difference–specific prevalence data [17]. Data on VCI prevalence are inconclusive, although VCI is a risk factor in progression to VaD and mortality [17]. Additional studies to update the current literature, especially using more unified definitions, would provide better understanding of demographics of VCI and VaD and contribute to enhancement of epidemiological knowledge for these pathologies. These studies should also address the severe lack of information on sex differences.
Risk factors that increase stroke and cardiovascular disease risk (e.g., age, hypertension, smoking, and diabetes) have been demonstrated to also increase risk of VaD [16, 21, 22]. A recent study reported myocardial infarction survivors having a higher risk for VaD [23]. Although overlap of stroke, cardiovascular disease, and VaD risk factors are generally agreed upon, controversies exist in terms of likelihood of shared risk factors between AD and VaD [24], as well as female sex as a VaD risk factor post-stroke [17, 22]. Reported risk factors for vascular contributions to cognitive impairment and dementia that increase risk in females only include delayed hormone replacement therapy, menopause, and preeclampsia [25]. Risk factors that were exclusive for males include heart disease and myocardial infarction, and factors that disproportionally increase vascular contributions to cognitive impairment and dementia risk include diabetes, midlife obesity, and hypertension being higher in females whereas stroke and hyperlipidemia being higher in males [25].
Etiology and pathology
Similar to the challenges in gathering data on demographics and risk factors, VCI etiology and pathology is multi-faceted and complex due to the nature of VCI and the presence/absence of progression to VaD. VCI involves certain brain regions (e.g., cortex, hippocampus), and patients typically present with neurological signs and symptoms including cognitive deficits (e.g., mental slowing, memory impairment, higher-order cognitive dysfunction: planning, organization, etc.) and behavioral/psychological symptoms (e.g., anxiety, depression, apathy) [4], which can be caused by various etiologies. Common etiologies of VCI and VaD include those of stroke (e.g., large-artery atherosclerosis, small vessel disease, myogenic stroke disorders, cardiovascular disease, etc.) and other mechanisms (e.g., oxidative stress, amyloid angiopathies, metabolic disorders, etc.) [2, 3, 24, 26–28]. Various animal models of VaD incorporate these etiologies and pathologies (e.g., vessel occlusion models, multiple infarct, and thromboembolism models), while some models focused on risk factors (e.g., high-fat diet, diabetes, spontaneous hypertensive rats stroke prone) [28]. Studies have reported that AD, VaD, and mixed dementia (combination of AD and VaD) evolve similarly in regard to disease progression [29]. When assessing cognitive domains, AD and VaD evolve differently in terms of memory impairment, depending on severity [29]. Other studies focusing on reversibility of VCI reported that patients occasionally return to normal cognition when the etiologies were in the context of acute stroke, autoimmune disorders, and heart failure; in addition, reversibility was observed in less than 20% of patients post-stroke with chance of recovery highest when soon after stroke event [3, 30].
Prevention and current treatments
Current strategies for the prevention of VCI and VaD involve eliminating risk factors (e.g., cardiovascular disease–related factors: hypertension, diabetes, smoking, atherosclerosis) and increasing physical exercise [1, 21]. Additional strategies include maintaining cognitive health and secondary prevention such as stroke prevention and management (e.g., early diagnosis and treatment of stroke, prevent reoccurrence, and slow progression of brain injury/damage post-stroke) [1, 21]. Although there is currently no specific treatments for VaD, therapeutic regimens target similar categories as seen with prevention: prevention of cardiovascular disease progression (e.g., statins, anti-hypertensives, exercise) and symptomatic relief (e.g., N-methyl-D-aspartate antagonists, cholinergic agents, oxidative stress-reducing agents) [19, 28, 31]. Information on prevention and current treatment in the literature is still fairly limited and have not taken into account sex differences in either prevention strategies or treatment responses. The next section will explore concepts of estrogen receptors and brain aging that will aid in this discussion of sex differences in VCI and VaD.
Estrogen receptors and brain aging
Estrogen receptors
Subtypes
An expanding body of evidence in regard to increased prevalence of various diseases in postmenopausal women has sparked a growing initiative in studying sex differences in cardiovascular disease, particularly sex hormones and mediation through respective receptors. In females, the most prevalent sex hormone studied is estrogen, whose effects are primarily mediated through estrogen receptors (ERs), and is involved in important reproductive and non-reproductive functions [32]. There are several ER subtypes: classical nuclear receptors (ER-alpha, ERα and ER-beta, ERβ) mediating predominantly genomic effects and those intracellular (G protein-coupled estrogen receptor 1, GPER) or membrane-bound (e.g., subset of ERα, ERβ, GPER) mediating more rapid, non-genomic effects [33, 34]. Most of the literature has focused on characterization of ERα and ERβ, which are encoded by the Esr1 and Esr2 gene, respectively; however, research on the role of GPER has been gaining momentum in recent years especially in the context of neuroprotective and cardiovascular effects [32, 33, 35]. The following sections will further explore these aspects of estrogen receptor changes due to aging and their involvement in VCI and VaD.
Changes due to aging
As with many other physiological processes, alterations of estrogen receptors occur due to aging, particularly changes in distribution, expression, and activity [36, 37]. These are important considerations to have in mind when investigating ER involvement in pathogenesis and treatment development. Estradiol effects on memory have been found to be not only hormone level dependent, but also dependent on the interaction via the various ER subtypes [36]. Although the exact mechanisms remain unclear, the loss of cognitive enhancing effects of estrogens, specifically estradiol (E2), have been linked to these age-related changes in estrogen receptor expression and signaling [36, 38]. A summary of the major findings related to aging are shown in Table 1. Changes in ER distribution and expression will subsequently be elaborated on in the context of brain region- and cerebrovascular-specific findings.
Table 1.
Summary of estrogen receptor expression changes during aging. OVX ovariectomized, E2 estradiol, SON supraoptic nucleus
| ER subtype, location | Experimental group | Methods | Main finding(s) | Reference |
|---|---|---|---|---|
| ERα and ERβ, brain | Female Sprague-Dawley rats; intact vs. OVX E2-treated; young (3–4 months), middle-aged (11–12 months), old (19–24 months) | In situ hybridization for ERα and ERβ, quantify mRNA levels |
ERα: mRNA in periventricular preoptic, medial preoptic, ventromedial, and arcuate nuclei with levels decreased in only periventricular preoptic nucleus of old rats ERβ: mRNA in various brain regions with a decrease in level only observed in the cerebral cortex and SON of OVX E2-treated middle-aged and old rats |
[39] |
| ERα, brain | Female Sprague-Dawley rats; young (3–4 months, n = 11) vs. aged (22–23 months, n = 10); OVX and E2 replacement implant | Postembedding immunogold and quantification | Subcellular distribution of ERα in the hippocampus and corresponding decreased responsiveness to E2 in female aged rats | [40] |
| ERα, cerebral blood vessels | Fischer 344 female rats; 3 months intact vs. OVX vs. OVX E2-treated | Immunohistochemistry, immunoblot/Western blot analyses |
Multiple forms of ERα identified and localized in both smooth muscle and endothelial cells of cerebral blood vessels in female rats All forms of cerebral blood vessel ERα decreased after OVX but increased after chronic E2 treatment |
[41] |
Brain region–specific changes
To better understand brain region–specific changes of estrogen receptors, it is important to note that during aging, the hypothalamic-pituitary-gonadal axis becomes increasingly less responsive to the regulatory feedback effects of estradiol. Although the underlying mechanisms for this decreased responsiveness is not fully understood, some researchers attribute the diminished responsiveness to estradiol regulatory feedback mechanisms to decreased expression of ERα and ERβ. A study by Wilson et al. [39] conducted in intact and ovariectomized estradiol-treated rats found that ERα mRNA was expressed in periventricular preoptic, medial preoptic, ventromedial, and arcuate nuclei with levels only decreased in periventricular preoptic nucleus of old rats. In contrast, ERβ mRNA was observed in various brain regions with a decrease in level only observed in the cerebral cortex and supraoptic nucleus of ovariectomized estradiol-treated, middle-aged, and old rats [39]. Another study using microarray analysis to investigate age-related estrogen-responsive gene expression reported that, compared with young and middle-aged mice, the aged mice exhibited reduced estradiol-induced hippocampal transcription [42]. Hippocampal changes during aging in regard to estrogen receptors were also supported by other studies including one conducted by Adams et al. [40] in female aged rats showing changes in subcellular distribution of ERα in the hippocampus and corresponding decreased responsiveness to E2.
Cerebrovascular-specific changes
While region-specific and age-related changes of estrogen receptors, especially in regions important in the pathogenesis of VCI and VaD (e.g., cerebral cortex, hippocampus), are important, it is also essential to consider cerebrovascular changes. It is well known that estrogen receptors mediate vasodilatory effects of estradiol and that there are various ER subtypes (e.g., ERα, ERβ, GPER) expressed throughout the vasculature [33, 43]. However, less is known about cerebrovascular-specific changes of estrogen receptors during aging. Despite data on age-related changes of estrogen receptors in the vasculature being inconclusive, a study by Stirone et al. [41] found ERα expression increased after chronic estradiol exposure but decreased in cerebral vessels after ovariectomy. Ovariectomizing rats is commonly used to model the postmenopausal female state, thus providing a bit of insight on cerebrovascular-specific changes of ER in the aging female. Several studies also suggest that age-related changes (e.g., distribution, expression, signaling activity) could alter vascular response to hormone replacement therapy [44]. This, again, is an important aspect when considering treatment options for patients, which will be further discussed in the next sections.
Estrogen receptor involvement in VCI and VaD
ER and VCI/VaD pathogenesis
Integrating what has been reviewed so far in terms of VCI and VaD epidemiological data and estrogen receptors in the aging brain, we can better appreciate the upcoming discussion on ER involvement in VCI and VaD pathogenesis. A significant amount of the literature addressing this topic has been focused on estrogen receptor gene polymorphisms (see Table 2). Studies on ERα gene polymorphisms (PvuII and XbaI) in VaD and AD patients found that the data suggest that ERα gene may be specific for and pose additional risk in late-onset AD patients [13]. Findings by Yaffe et al. [45] support the association of the same ESR1, or ERα, polymorphisms with risk in cognitive impairment development in a study consisting of 2625 women, 65 years or older. ERβ gene polymorphisms have also been reported to have association with increased risk for VaD in elderly Jewish women (ESR2 rs4986938) [15] and Chinese Han women (ESR2 rs944050) [14]; however, authors of both studies commented on the need of future studies to confirm findings. Studies also support ERα and ERβ gene polymorphisms in association for increased risk for cognitive impairment, although further clinical evaluation was needed in some studies to determine if the cognitive impairment was due to AD or vascular etiologies [46, 47]. Apart from the studies on ER gene polymorphisms, other data related to ER and VCI/VaD pathogenesis involve ER and cardiovascular disease; however, limited information exists for ER role in cerebrovascular etiologies [33, 44].
Table 2.
Summary of estrogen receptor gene polymorphisms relating to vascular dementia
| ER subtype, gene | Polymorphism/SNPs examined | Ethnicity | Main finding(s) | Reference |
|---|---|---|---|---|
| ERα, ESR1 | PvuII and XbaI | – | No significant difference in VaD patients vs. controls; significantly increased in late-onset AD | [13] |
| ERβ, ESR2 | ESR2 rs4986938 | Jewish | Association of ESR2 rs4986938 and VaD in elderly women (n = 60; age 82 ± 6) | [15] |
| ERβ, ESR2 | ESR2 rs944050 and ESR2 rs4986938 | Chinese Han | Association of ESR2 rs944050 and increased risk of VaD in women (n = 61; age > 50) | [14] |
ER and VCI/VaD treatments
Review of current treatment for VCI and VaD in “Prevention and current treatments” section identified two major categories: (1) prevention of cardiovascular disease/stroke and (2) symptomatic relief. Although current treatment options do not specifically mention estrogen receptors as targets, studies in this avenue can provide insight for potential therapeutics as estrogen receptors are involved in aspects of cardioprotection and neuroprotection. Studies have suggested cardioprotective roles for estradiol mediated by estrogen receptors, although the specific ER subtype underlying these mechanisms remains unclear [48]. A selective estrogen receptor modulator, bazedoxifene, reduced the incidence of cerebral aneurysm rupture in ovariectomized rats [49]. Zhu et al. [50] reported that low-dose E2 replacement has potential to attenuate negative neurological consequences in an animal model of VaD.
In addition to benefits in vascular pathologies, the literature for neuroprotective effects mediated by ER, particularly GPER, is greatly expanding [51]. Tang et al. [52] provided evidence that suggests that GPER mediates rapid signaling and neuroprotective effects of E2 in the hippocampus in a rat model of global cerebral ischemia. Neuroprotection of E2 mediated by GPER was also demonstrated, suggesting that early activation of GPER improved cognitive outcomes induced by traumatic brain injury via the PI3K/Akt pathway [53]. Additionally, GPER expressed in microglia may mediate the anti-inflammatory effects of E2 after ischemic stroke [54].
Conclusions
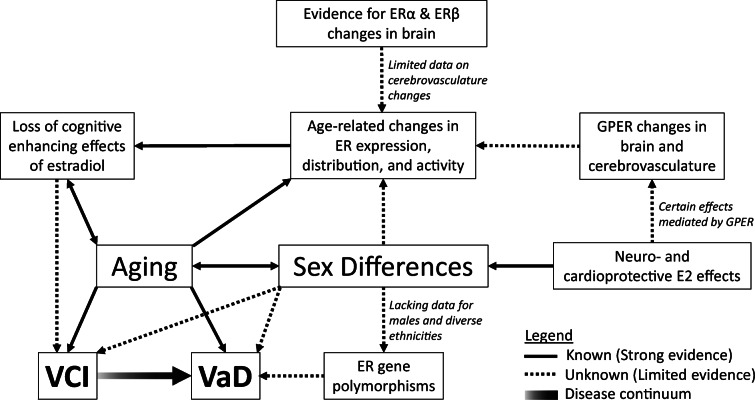
Research in vascular cognitive impairment and vascular dementia is valuable, especially with the growing aging population. However, literature in the field is complicated due to varying definitions of VCI and VaD. With more consistent definitions, the literature in the field can be tightened, allowing for more studies to help close the knowledge gap in the less known aspect of VCI/VaD sex differences, particularly estrogen receptor involvement. Reports on association of ER gene polymorphisms with increased risk of VaD and cardioprotective/neuroprotective effects of estrogen mediated by various ER subtypes offer some, though limited, understanding. Aside from having researchers use the same operational definition of VCI/VaD, additional studies investigating more explicitly the role of estrogen receptor expression and their changes during aging in the cerebrovasculature will need to be conducted in order to improve the current literature on VCI/VaD and sex differences. Some salient, suggested studies to investigate include (1) detailing the expression of various ER subtypes in VCI/VaD-related brain regions and cerebrovasculature of young and aging females (e.g., postmenopausal, ovariectomized, hormone replacement, reproductive senescent), (2) determining sex differences at the intersection between blood flow and neurodegeneration, (3) defining the mechanisms of ER contributions beyond association of ER gene polymorphisms, and (4) testing GPER involvement in VCI/VaD and inclusion in treatment development (Fig. 1). In conclusion, further research in estrogen receptor involvement in VCI/VaD is an avenue worth exploring as this will provide additional insight for potential mechanisms in pathogenesis and novel therapeutic approaches for VCI and VaD.
Fig. 1.
Summary of the proposed relationships among estrogen receptors, aging, vascular cognitive impairment (VCI), and vascular dementia (VaD) highlighting areas requiring additional research. Solid lines indicate areas with strong support from the existing literature. Dotted lines represent areas of research or relationships proposed to be existing knowledge gaps. E2 estradiol, ER estrogen receptor, ERα estrogen receptor alpha, ERβ estrogen receptor beta, GPER G protein-coupled estrogen receptor 1
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the National Institutes of Health/National Institute on Aging T32 grant AG020494 and R01 HL142341.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Erkinjuntti T, Roman G, Gauthier S, Feldman H, Rockwood K. Emerging therapies for vascular dementia and vascular cognitive impairment. Stroke. 2004;35(4):1010–1017. doi: 10.1161/01.STR.0000120731.88236.33. [DOI] [PubMed] [Google Scholar]
- 2.Roman GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, Lopez-Pousa S, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226(1–2):81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120(3):573–591. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- 4.van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, Scheltens P. Vascular cognitive impairment. Nat Rev Dis Primers. 2018;4:18003. doi: 10.1038/nrdp.2018.3. [DOI] [PubMed] [Google Scholar]
- 5.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis. 2015;241(1):211–218. doi: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97(1):1–37. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 7.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 8.Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry. 2016;6(1):54–65. doi: 10.5498/wjp.v6.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Singh M. Sex differences in cognitive impairment and Alzheimer’s disease. Front Neuroendocrinol. 2014;35(3):385–403. doi: 10.1016/j.yfrne.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15(5):451–452. doi: 10.1016/S1474-4422(16)00067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson RS, Weir DR, Leurgans SE, Evans DA, Hebert LE, Langa KM, Plassman BL, Small BJ, Bennett DA. Sources of variability in estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimers Dement. 2011;7(1):74–79. doi: 10.1016/j.jalz.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji Y, Urakami K, Wada-Isoe K, Adachi Y, Nakashima K. Estrogen receptor gene polymorphisms in patients with Alzheimer’s disease, vascular dementia and alcohol-associated dementia. Dement Geriatr Cogn Disord. 2000;11(3):119–122. doi: 10.1159/000017224. [DOI] [PubMed] [Google Scholar]
- 14.Xin J, Zhang J, Gao Y, Xiong L. Association of estrogen receptor beta gene polymorphisms with vascular dementia in women. Neurol Sci. 2012;33(5):1029–1035. doi: 10.1007/s10072-011-0885-9. [DOI] [PubMed] [Google Scholar]
- 15.Dresner-Pollak R, Kinnar T, Friedlander Y, Sharon N, Rosenmann H, Pollak A. Estrogen receptor beta gene variant is associated with vascular dementia in elderly women. Genet Test Mol Biomarkers. 2009;13(3):339–342. doi: 10.1089/gtmb.2008.0129. [DOI] [PubMed] [Google Scholar]
- 16.Smith EE. Clinical presentations and epidemiology of vascular dementia. Clin Sci (Lond) 2017;131(11):1059–1068. doi: 10.1042/CS20160607. [DOI] [PubMed] [Google Scholar]
- 17.Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol. 2019;73(25):3326–3344. doi: 10.1016/j.jacc.2019.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebert R, Brayne C. Epidemiology of vascular dementia. Neuroepidemiology. 1995;14(5):240–257. doi: 10.1159/000109800. [DOI] [PubMed] [Google Scholar]
- 19.Rockwood K. Vascular cognitive impairment and vascular dementia. J Neurol Sci. 2002;203–204:23–27. doi: 10.1016/s0022-510x(02)00255-1. [DOI] [PubMed] [Google Scholar]
- 20.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorelick PB, Counts SE, Nyenhuis D. Vascular cognitive impairment and dementia. Biochim Biophys Acta. 2016;1862(5):860–868. doi: 10.1016/j.bbadis.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 23.Sundboll J, Horvath-Puho E, Adelborg K, Schmidt M, Pedersen L, Botker HE, et al. Higher risk of vascular dementia in myocardial infarction survivors. Circulation. 2018;137(6):567–577. doi: 10.1161/CIRCULATIONAHA.117.029127. [DOI] [PubMed] [Google Scholar]
- 24.Javanshiri K, Waldo ML, Friberg N, Sjovall F, Wickerstrom K, Haglund M, et al. Atherosclerosis, hypertension, and diabetes in Alzheimer’s disease, vascular dementia, and mixed dementia: prevalence and presentation. J Alzheimers Dis. 2018;65(4):1247–1258. doi: 10.3233/JAD-180644. [DOI] [PubMed] [Google Scholar]
- 25.Gannon OJ, Robison LS, Custozzo AJ, Zuloaga KL. Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem Int. 2019;127:38–55. doi: 10.1016/j.neuint.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 26.Du SQ, Wang XR, Xiao LY, Tu JF, Zhu W, He T, et al. Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol. 2017;54(5):3670–3682. doi: 10.1007/s12035-016-9915-1. [DOI] [PubMed] [Google Scholar]
- 27.Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018;134(Pt B):226–239. doi: 10.1016/j.neuropharm.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowler JV, Eliasziw M, Steenhuis R, Munoz DG, Fry R, Merskey H, Hachinski VC. Comparative evolution of Alzheimer disease, vascular dementia, and mixed dementia. Arch Neurol. 1997;54(6):697–703. doi: 10.1001/archneur.1997.00550180021007. [DOI] [PubMed] [Google Scholar]
- 30.Rasquin SM, Lodder J, Verhey FR. Predictors of reversible mild cognitive impairment after stroke: a 2-year follow-up study. J Neurol Sci. 2005;229–230:21–25. doi: 10.1016/j.jns.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292(23):2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 32.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116(3):561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol. 2012;86(10):1491–1504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist. 2014;20(5):534–545. doi: 10.1177/1073858413519865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Segura LM, Diz-Chaves Y, Perez-Martin M, Darnaudery M. Estradiol, insulin-like growth factor-I and brain aging. Psychoneuroendocrinology. 2007;32(Suppl 1):S57–S61. doi: 10.1016/j.psyneuen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22(4):656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123(6):593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 40.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, et al. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22(9):3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Physiol Endocrinol Metab. 2003;284(1):E184–E192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- 42.Aenlle KK, Foster TC. Aging alters the expression of genes for neuroprotection and synaptic function following acute estradiol treatment. Hippocampus. 2010;20(9):1047–1060. doi: 10.1002/hipo.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reslan OM, Yin Z, do Nascimento GR, Khalil RA. Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat. J Cardiovasc Pharmacol. 2013;62(1):26–40. doi: 10.1097/FJC.0b013e31828bc88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khalil RA. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmacol. 2013;86(12):1627–1642. doi: 10.1016/j.bcp.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaffe K, Lui LY, Grady D, Stone K, Morin P. Estrogen receptor 1 polymorphisms and risk of cognitive impairment in older women. Biol Psychiatry. 2002;51(8):677–682. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]
- 46.Olsen L, Rasmussen HB, Hansen T, Bagger YZ, Tanko LB, Qin G, et al. Estrogen receptor alpha and risk for cognitive impairment in postmenopausal women. Psychiatr Genet. 2006;16(2):85–88. doi: 10.1097/01.ypg.0000194445.27555.71. [DOI] [PubMed] [Google Scholar]
- 47.Yaffe K, Lindquist K, Sen S, Cauley J, Ferrell R, Penninx B, Harris T, Li R, Cummings SR. Estrogen receptor genotype and risk of cognitive impairment in elders: findings from the Health ABC study. Neurobiol Aging. 2009;30(4):607–614. doi: 10.1016/j.neurobiolaging.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. 2017;8(1):33. doi: 10.1186/s13293-017-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maekawa H, Tada Y, Yagi K, Miyamoto T, Kitazato KT, Korai M, Satomi J, Hashimoto T, Nagahiro S. Bazedoxifene, a selective estrogen receptor modulator, reduces cerebral aneurysm rupture in Ovariectomized rats. J Neuroinflammation. 2017;14(1):197. doi: 10.1186/s12974-017-0966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Y, Zhang Q, Zhang W, Li N, Dai Y, Tu J, Yang F, Brann DW, Wang R. Protective effect of 17beta-estradiol upon hippocampal spine density and cognitive function in an animal model of vascular dementia. Sci Rep. 2017;7:42660. doi: 10.1038/srep42660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu CL, Herndon C. New roles for neuronal estrogen receptors. Neurogastroenterol Motil. 2017;29(7). 10.1111/nmo.13121. [DOI] [PubMed]
- 52.Tang H, Zhang Q, Yang L, Dong Y, Khan M, Yang F, Brann DW, Wang R. GPR30 mediates estrogen rapid signaling and neuroprotection. Mol Cell Endocrinol. 2014;387(1–2):52–58. doi: 10.1016/j.mce.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang ZF, Pan ZY, Xu CS, Li ZQ. Activation of G-protein coupled estrogen receptor 1 improves early-onset cognitive impairment via PI3K/Akt pathway in rats with traumatic brain injury. Biochem Biophys Res Commun. 2017;482(4):948–953. doi: 10.1016/j.bbrc.2016.11.138. [DOI] [PubMed] [Google Scholar]
- 54.Zhao TZ, Ding Q, Hu J, He SM, Shi F, Ma LT. GPER expressed on microglia mediates the anti-inflammatory effect of estradiol in ischemic stroke. Brain Behav. 2016;6(4):e00449. doi: 10.1002/brb3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]