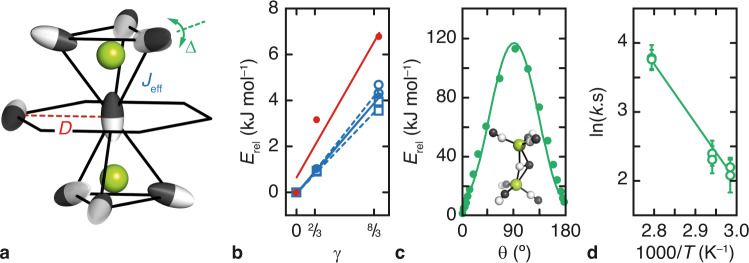
Fig. 3. Microscopic single-ion and pairwise interaction parameters in Cd(CN)2.
a The effective exchange interaction (strength ) operates between nearest neighbours within the same pyrochlore sublattice. Dipolar interactions (strength ) give rise to an effective exchange interaction between nearest neighbours on alternate sublattices. The single-ion anisotropy term reflects the enthalpy barrier to CN– reorientations. b Relative DFT energies for a series of single network (filled blue symbols) and interpenetrated (filled red symbols) Cd(CN)2 configurations with different CdCnN4–n coordination environments: CdC2N2 (geometric parameter ), CdC3N/CdCN3 () and CdC4/CdN4 (). Simple geometric arguments give and for single-network and interpenetrated Cd(CN)2, respectively. The corresponding NMR-derived values are shown with open blue symbols (squares = ref. 34 circles = this study). c Nudged elastic band calculation energies for a 180° CN– orientation flip (filled green symbols) and corresponding fit (solid green line), from which the value of was obtained. The 90° transition state involves a C-bridged Cd–(CN)–Cd linkage (inset) and a reduced Cd…Cd separation. d Arrhenius plot reflecting the temperature dependence of CN– flipping rates as determined using 113Cd EXSY spectroscopy. Error bars denote the standard error in rate constants determined extracted during fitting (see Supplementary Figure 6 and Supplementary Table 7). The experimental value of is given by the gradient of the linear fit (solid line).