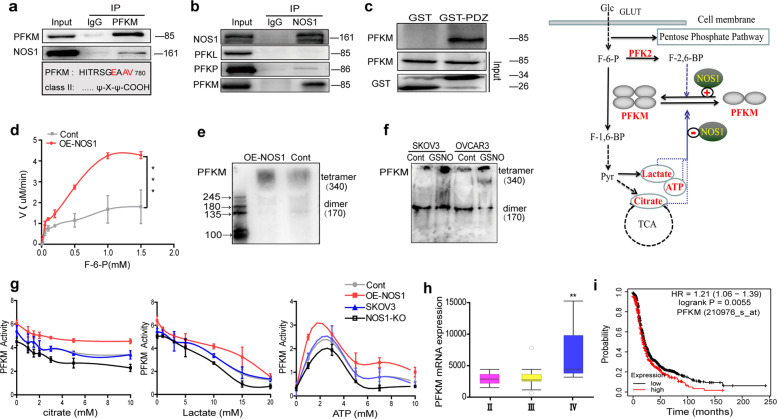
Fig. 2. NOS1 enhances its activity through S-nitrosation of PFKM.
a Co-immunoprecipitation (Co-IP) detected whether PFKM with NOS1 could bind in SKOV3-OE-NOS1 cells by using PFKM monoclonal antibody (upon); PFKM, one proposed NOS1 binding partner, display a class II PDZ domain-binding motif at its C termini (down). b Co-IP detected whether NOS1 with PFKL, PFKP, and PFKM could bind in SKOV3-OE-NOS1 cells by using NOS1 monoclonal antibody. c Western blot results showed that PDZ domain of GST-tagged could bind to PFKM by GST pull-down assay. d Enzyme kinetics assay displayed the Km (the concentration of F-6-P required to reach half-maximal activation) decreased significantly and the Vmax (maximum velocity) increased significantly in the SKOV3-OE-NOS1 cells (Km = 0.591 and 0.692, Vmax = 8.33 and 2.6 μM/min for OE-NOS1 and control cells respectively) (***P < 0.001, one-way ANOVA). e Western blotting by non-reducing SDS-polyacrylamide gel electrophoresis (SDS-PAGE) displayed the tetramer and dimer contents of PFKM in OE-NOS1 and control SKOV3 cells. f The tetramer and dimer contents of PFKM in SKOV3 and OVCAR3 cells treated with GSNO (1 mM) or PBS for 30 min respectively. g The activity of PFKM under different concentrations of citrate (left), Lactate (middle) and ATP (right) in SKOV3 vs. NOS1-KO cells and SKOV3-OE-NOS1 vs. control cells. h Boxplots correlating the stage of tumors and the mRNA expression levels of tumor glycolytic genes PFKM are shown in ovary cancer samples (GSE51373). (**p < 0.01, Student’s t-test). i Kaplan–Meier analyzed for the overall survival time between PFKM high or low expressed patients.