Figure 1.
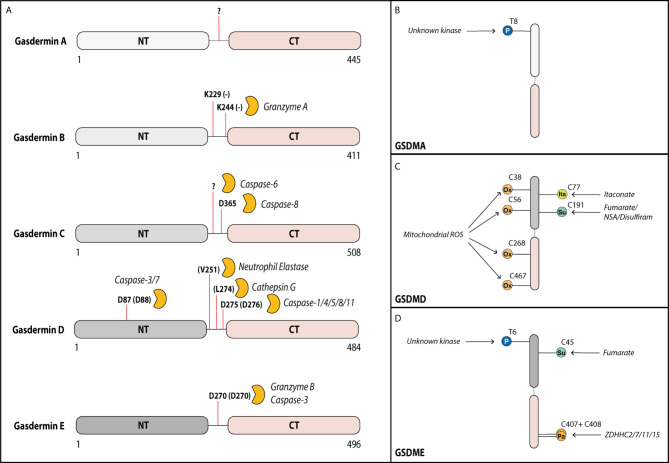
Gasdermins can be cleaved by various proteases in their linker region. Activity of gasdermins is regulated by cleavage and by post-translational modifications. (A) Gasdermin A can form membrane pores after cleavage of the linker domain, but the cleaving protease remains unknown. In tumor cells, Gasdermin B can be cleaved by granzyme A from cytotoxic T cells at Lys229 and Lys244 into the pore forming fragment. In several cell types including most myeloid cells, gasdermin D can be cleaved by multiple proteases at Asp275 (mouse Asp276) leading to its activation, but it can additionally be cleaved at Asp87 (mouse Asp88) by caspase-3 and -7 inactivating it during apoptosis. In neutrophils, gasdermin D can also be cleaved by neutrophil elastase and cathepsin D. In response to some chemotherapy drugs, gasdermin C can be cleaved by caspase-6 (at unknown site) and caspase-8 (at Asp365) into the pore forming fragment. Gasdermin E can be cleaved by granzyme B or caspase-3 at Asp270 leading to activation. Activity of gasdermins is also regulated by several post-translational modifications. (B) Gasdermin A can be phosphorylated (P) by an unknown kinase at Thr8, supporting its pore-forming capacity. (C) Gasdermin D is oxidized (Ox) at multiple residues (Cys38, Cys56, Cys268 and Cys467) by reactive oxygen species from the mitochondria promoting its activation. Prolonged LPS exposure of macrophages results in binding of itaconate at Cys77 preventing gasdermin D cleavage. Gasdermin D can also be succinated (Su) at Cys191 by the metabolic product fumarate or by covalent binding of the cysteine-reactive drugs necrosulfonamide (NSA) or disulfiram, which prevents its oligomerization. (D) Similar to gasdermin A, gasdermin E is phosphorylated at Thr6 promoting its pore formation. Gasdermin E is inhibited by succination at Cys45. During the activation, the palmitoyltransferases ZDHHC2, -7, -11 and -15 palmitoylate (Pa) gasdermin E at Cys407 and Cys408 promoting the dissociation of the GSDME-NT from GSDME-CT.