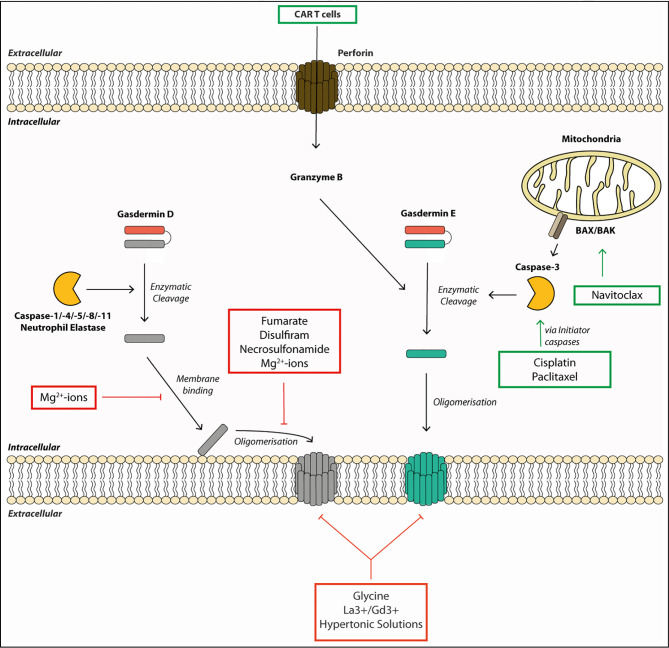
Figure 2.
Gasdermin pore formation and cell lysis require multiple steps and can be targeted by therapeutics. Gasdermins are activated by enzymatic cleavage by proteases such as caspases or neutrophil elastase. This liberates the pore-forming N-terminal fragment (GSDM-NT). The GSDM-NT binds to phospholipids on the inner membrane leaflet. GSDM-NT then oligomerizes to form a membrane pore allowing the efflux of small proteins and ions across the membrane. Gasdermin pores eventually lead to cell death and membrane rupture. Some drugs promote gasdermin-mediated cell death. For example, chemotherapeutic drugs, such as Cisplatin, Paclitaxel or Navitoclax can initiate gasdermin E cleavage. They activate the initiator caspases, which, in turn, lead to gasdermin E cleavage by activating the executioner caspase-3. Other drugs or small molecules can block gasdermin-mediated cell death. For example, the membrane binding and the oligomerization step of gasdermin D can be blocked by Mg2+-ions by an unknown mechanism. Fumarate, Necrosulfonamide and Disulfiram can block oligomerization of gasdermin D by modifying Cys191. Finally, membrane rupture can be blocked by the osmoprotectant glycine, hypertonic solutions or the lanthanide ions La3+ and Gd3+.