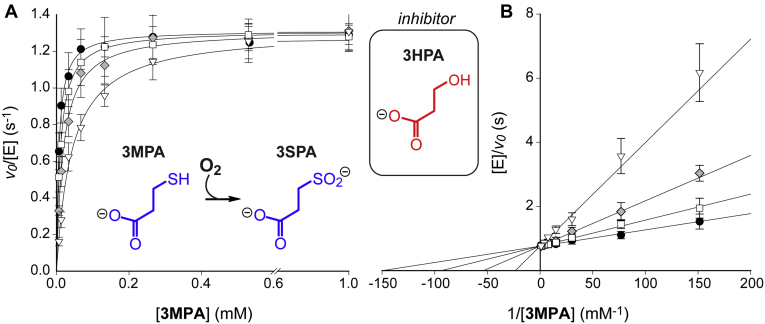
Figure 3.
3-hydroxypropionic acid (3HPA) inhibition of Av3MDO-catalyzed 3MPA-reactions. Kinetic data were collected in the presence of 167 μM (white square), 500 μM (gray diamond), and 1500 μM (white triangle) 3HPA for comparison to the uninhibited enzyme (black circle). SigmaPlot was used to globally fit enzyme kinetics in either Michaelis–Menten (A) or Lineweaver–Burk (B) fashion assuming a fully competitive model of inhibition. The resulting least-square fits (solid-lines) are overlaid on kinetic data to obtain values for kcat, KM, and KI, as well as the error associated with each parameter [1.31 ± 0.01 s−1, 6.7 ± 0.4 μM, and 280 ± 26 μM, respectively]. Michaelis–Menten results (A) are presented with a gap ranging from 0.6 to 0.8 mM 3MPA to avoid data crowding at low concentration.