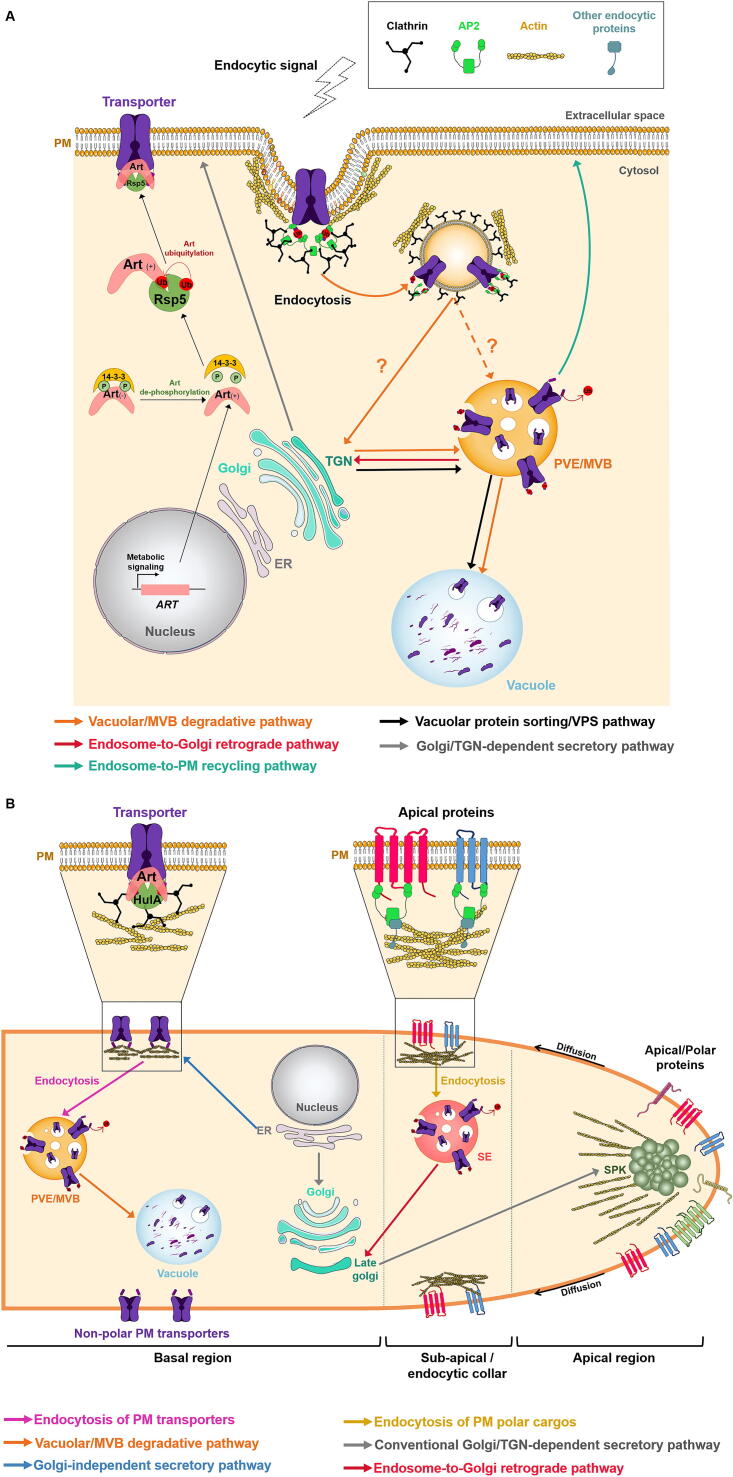
Fig. 1.
Overview of endocytosis and main trafficking pathways in budding yeast and filamentous fungi. (A) Clathrin-mediated endocytosis and main trafficking pathways of plasma membrane proteins in budding yeast (adapted from [6], [7], [8], [9], [10], [57], [58], [59], [60]). Environmental changes, stress or specific compounds (endocytic signals) can trigger PM nutrient transporter endocytosis, a process normally preceded by PM transporter ubiquitylation, mediated by Rsp5-ART complexes, and dependent on clathrin and on the AP2 complex. According to the Day et al. recent model [6], cargo proteins internalized into endocytic vesicles are sorted to the TGN (which is proposed to also serve as an early and recycling endosome). They are then delivered to the PVE/MVB, where cargo can i) be recycled back to the PM (endosome-to-PM recycling pathway); ii) be directed to the endosome-to-Golgi retrograde trafficking pathway and be secreted and recycled back to the PM, via the secretory pathway; iii) be targeted for vacuolar degradation by the vacuolar/MVB degradative pathway. It is still unclear if some endocytic vesicles can be targeted directly from the PM to the PVE/MVB.
Newly-synthetized PM transporters at the ER are thought to be targeted from the Golgi and can then be sorted either to: i) the PM via the secretory pathway, or ii) to the vacuole indirectly, via vacuolar protein sorting pathway or iii) directly to the vacuole via alkaline phosphatase pathway. The latter pathway is not explored in the context of this review, so it will not be further detailed. (B)Endocytosis and main trafficking pathways of plasma membrane transporters and polar proteins in Aspergillus nidulans (adapted from [54], [55], [56]). In A. nidulans , there are two distinct endocytic pathways. The pathway required for the internalization of PM transporters involves their ubiquitylation by HulARsp5 -Art complexes, and depends on clathrin but not on AP2. All internalized transporters studied, so far, are degraded in the vacuole via the MVB degradative pathway. The other endocytic route, essential for polar growth, is for apical PM proteins that diffuse laterally to the sub-apical/endocytic collar (enriched in actin patches), where they are internalized by a clathrin-independent, but AP2-dependent process. Internalized vesicles are targeted to sorting endosomes (SE), then to the late Golgi/TGN, via endosome-to-Golgi retrograde pathway. From this point, AP1/clathrin coated-vesicles transport polar cargo to the so-called Spitzenkörper (SPK), from which polar proteins fuse to the PM. Additionally, two different secretory pathways were also described [53], [55]. While polar proteins follow the conventional Golgi-to-TGN dependent secretory pathway, newly synthetized transporter proteins traffic from the ER to the PM by an alternative pathway, without passing through the Golgi. ER, endoplasmic reticulum; MVB, multi vesicular bodies; PVE, pre-vacuolar endosome; PM, plasma membrane; TGN, trans-Golgi network; SE, sorting endosomes; SPK, Spitzenkörper; signals (+) and (-) represent activation and inhibition, respectively.