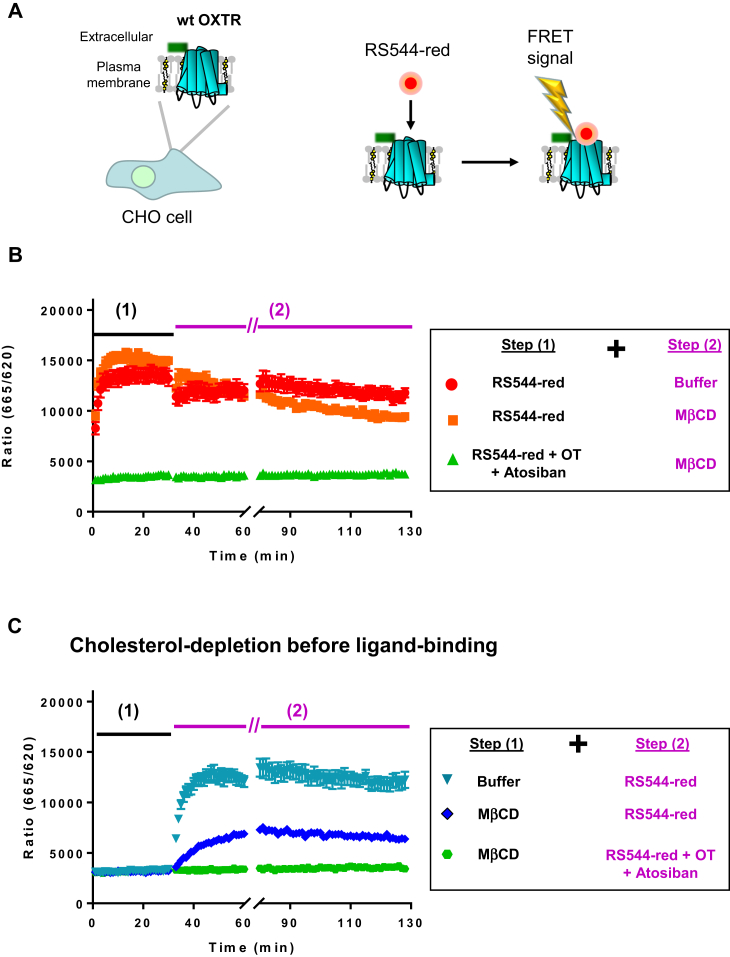
Fig. 8.
The activity of wild-type OXTR is also preserved in the ligand-bound form during cholesterol depletion in mammalian cells. A: Diagram showing wild-type OXTR heterologously expressed in CHO cells. The receptor has a SNAP tag in N-terminus that binds the cryptate of terbium, Lumi4-Tb. The fluorescent antagonist RS544-red binds to the receptor and generates a FRET signal that is recorded in real-time (time-resolved FRET, TR-FRET). B: TR-FRET signal (ratio of light emission intensity at 665 nm over the intensity at 620 nm) as a function of time in the indicated conditions. Each point is an average of 12 measurements. Cells are placed in wells in 96-well plates and incubated for 30 min with solutions indicated in step 1 in the legend. The solutions of Step 2 are added to the wells for additional incubation of 30 min + 60 min. To avoid dynamic and unspecific FRET, RS544-red was used at 20 nM. The concentration of MβCD was increased to 40 mM because of the shorter incubation time (30 min). Co-incubation with 5 μM of unlabeled Atosiban and Oxytocin were used to determine the level of nonspecific FRET. C: Same conditions of experiments with the solutions of Step 1 and 2 indicated in the legend. MβCD, methyl-β cyclodextrin; OXTR, oxytocin receptor.