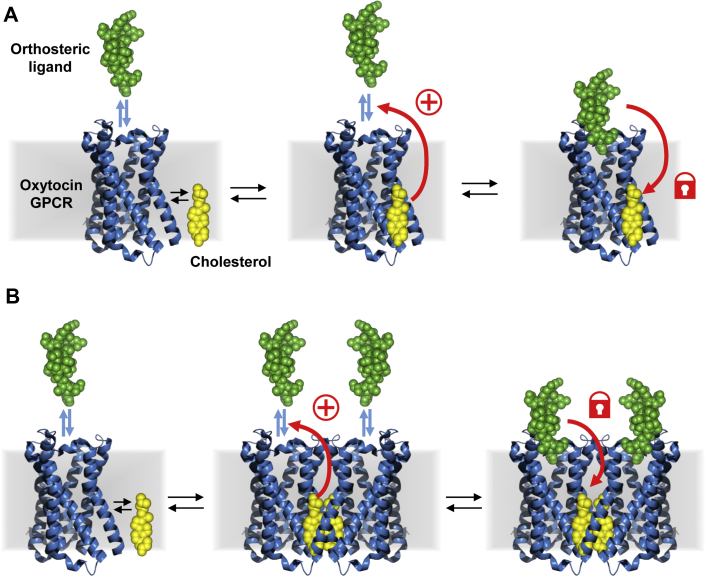
Fig. 9.
Monomeric and dimeric models of cholesterol sequestration by ligand-bound OXTR. A: Diagram showing the monomeric model of cholesterol sequestration by ligand-bound OXTR. Cholesterol and ligand binding on OXTR is in equilibrium between bound and unbound forms. Binding of cholesterol is known to induce a high-affinity state of OXTR for its ligands. In this model, binding of ligands will induce conformational changes that stabilize the binding of cholesterol and render it inaccessible to external chelators. In gray, the lipid membrane, in blue, the structure of a GPCR (M2, PDB code: 3uon) representing OXTR, in green an external orthosteric peptidic ligand (ET, PDB code: 5glh) and in yellow a cholesterol molecule (PDB code: 3d4s). B: The dimeric model showing the cholesterol-induced stabilization of OXTR homodimers which represent the high-affinity state of the receptor for its orthosteric ligands. In this model, binding of orthosteric ligands would stabilize the homodimeric form of OXTR and embed bound cholesterol molecules at the interface. GPCR, G protein-coupled receptor; OXTR, oxytocin receptor.