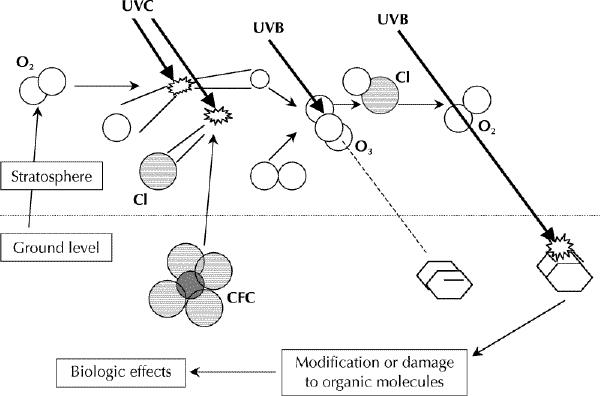
Fig. 1: Simplified scheme of how the stratospheric ozone layer is formed and absorbs ultraviolet B (UVB) radiation and how chlorofluorocarbons (CFCs) degrade the ozone layer and increase UVB-induced biological effects. Molecular oxygen (O2) and CFCs diffuse upward into the stratosphere, where they are decomposed by shortwave UV radiation (UVC). Atomic oxygen (O) recombines with molecular oxygen to form ozone (O3). Ozone absorbs the short wavelengths in the UVB band, blocking photochemical reactions in organic molecules. Reactive chlorine (Cl) released from CFCs catalyzes the breakdown of ozone to molecular oxygen, which does not absorb UVB radiation. More UVB radiation thus passes through the stratosphere, enhancing photochemical reactions in organic molecules and increasing corresponding biological effects.
