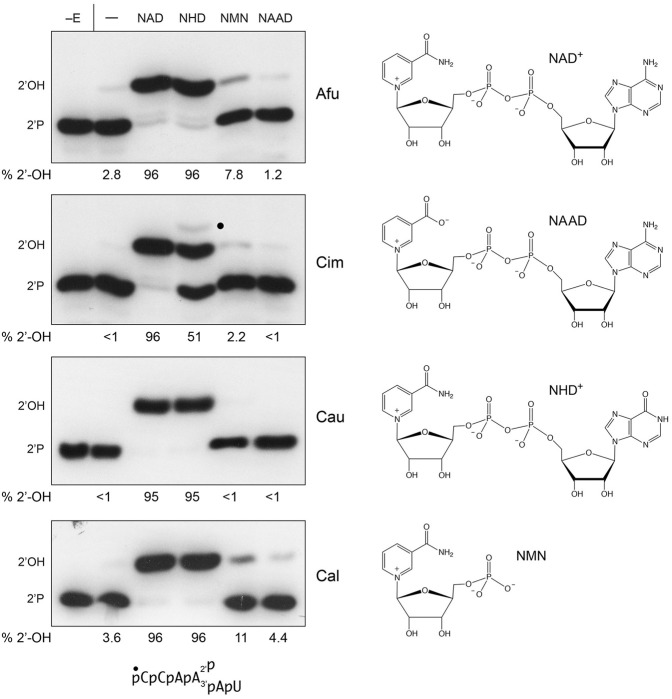
FIGURE 3.
NAD+ analogs as substrates for fungal Tpt1 enzymes. (Left panel) Reaction mixtures (10 µL) containing 100 mM Tris-HCl, pH 7.5, 2 mM DTT, 0.2 µM (2 pmol) 5′ 32P-labeled 6-mer 2-PO4 RNA (shown at bottom), 1 mM nicotinamide adenine dinucleotide (NAD), nicotinamide hypoxanthine dinucleotide (NHD), nicotinamide mononucleotide (NMN), or nicotinic acid adenine dinucleotide (NAAD) as indicated above the lanes, and 10 fmol AfuTpt1, CimTpt1, CauTpt1, or CalTpt1 as indicated on the right were incubated at 37°C for 30 min. The reaction products were analyzed by urea-PAGE and visualized by autoradiography. Tpt1 was omitted from reactions shown in lanes −E. Control reactions containing Tpt1 but no NAD+ are included in lanes −. The species corresponding to the 2′-phosphate substrate and 2′-OH product are indicated on the left. The RNA-2′-phospho-HDPR intermediate detected in the CimTpt1 reaction with NHD is denoted by a dot. The extents of formation of the 2′-OH product (as percent of the total 32P-labeled RNA) are specified below the lanes. (Right panel) The chemical structures of the substrates are shown.