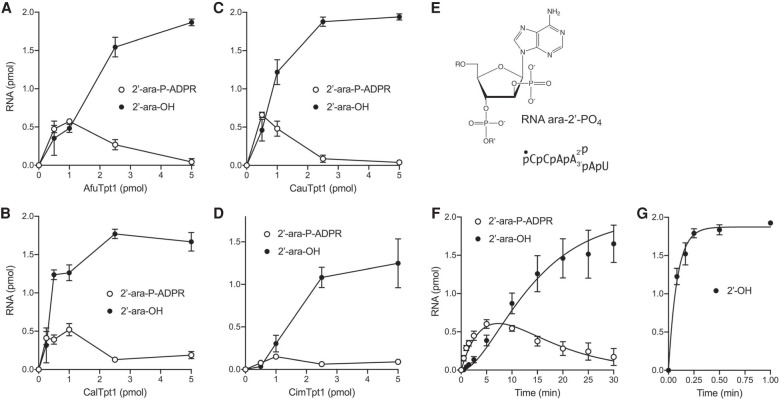
FIGURE 7.
Effect of an arabinose sugar at the 2′-phosphate branchpoint. (A–D) Tpt1 titrations. Reaction mixtures (10 µL) containing 100 mM Tris-HCl, pH 7.5, 2 mM DTT, 1 mM NAD+, 0.2 µM (2 pmol) 5′ 32P-labeled 6-mer ara-2′-PO4 substrate (shown in E, highlighting the chemical structure of the arabinose-2′-PO4 branchpoint), and AfuTpt1 (A), CalTpt1 (B), CauTpt1 (C), or CimTpt1 (D) as specified on the x-axes were incubated at 37°C for 30 min. The reaction products were analyzed by urea-PAGE and quantified by scanning the gels. The extents of formation of the 2′-OH product and the ADP-ribosylated intermediate are plotted as a function of input Tpt1. Each datum in the graphs is the average of three separate experiments ±SEM. (F,G) Single-turnover kinetics. Reaction mixtures (100 µL) containing 100 mM Tris-HCl, pH 7.5, 2 mM DTT, 1 mM NAD+, 0.2 µM 5′ 32P-labeled 6-mer ara-2′-PO4 substrate (F) or unmodified 6-mer RNA 2′-PO4 substrate (G), and 0.5 µM AfuTpt1 were incubated at 37°C. The reactions were initiated by adding enzyme to a prewarmed reaction mixture. Aliquots (10 µL, containing 2 pmol of RNA) were withdrawn at the times specified on the x-axis and quenched immediately with three volumes of cold 90% formamide, 50 mM EDTA. The extents of formation of the 2′-OH product and the ADP-ribosylated intermediate are plotted as a function of reaction time. Each datum is the average of three independent titration or time-course experiments (±SEM). The data in F were fit by nonlinear regression in Prism to a unidirectional two-step mechanism. The data in G were fit to a single exponential.