Fig. 3. δ-Secretase cleavage of UNC5C mediates T835 mutant’s cytotoxicity.
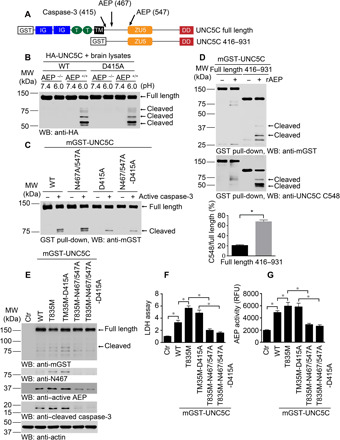
(A) Schematic diagram shows the cutting sites on UNC5C by caspase-3 and δ-secretase. IG, immunoglobulin-like domain; T, type I thrombospondin motifs; TM, transmembrane domain; ZU5, ZU5 domain; DD, death domain. (B) HA-UNC5C (WT and D415A) were incubated with brain lysates derived from WT (AEP+/+) or AEP KO (AEP−/−) mice at pH 7.4 or 6.0, respectively. WB showed that D415A mutation did not affect UNC5C cleaved by δ-secretase. (C) Purified mGST-UNC5C (WT, N467/547A, D415A, and N467/547A-D415A) were incubated with active caspase-3 in caspase assay buffer. WB showed that N467/547A mutation did not affect UNC5C cleaved by caspase-3. (D) Truncated UNC5C by caspase-3 (UNC5C 416–931) was prone to be cleaved by δ-secretase compared with full length, promoting more C-terminal fragments (death domain) production (means ± SEM; n = 3). **P < 0.01 by Student’s t test. (E) WB showed that UNC5C with T835M mutant was truncated into fragments when expressed in SH-SY5Y cells, inducing cell death. δ-Secretase cleavage of T835M was indispensable for UNC5C to mediate cell death. (F) Lactate dehydrogenase (LDH) assay showed the cytotoxicity of above mutated UNC5C (means ± SEM; n = 3). (G) δ-Secretase activity assay (means ± SEM; n = 3). *P < 0.05 by one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test for (F) and (G).
