Fig. 9. A schematic illustration of molecular mechanisms by which SRSF1 regulates the late stage of thymocyte development.
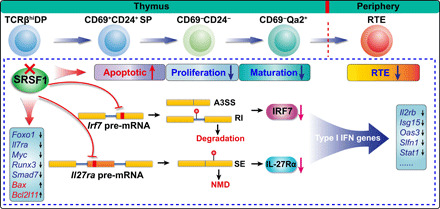
The continuous processes of postselection thymocyte maturation before egression to periphery are shown on the top of the diagram. The red cross mark represents the deletion of SRSF1 in TCRβhi DP thymocytes. The accelerated apoptosis, impaired proliferation, blockade of terminal maturation, and notably diminished RTEs in SRSF1-deficient mice are exhibited with arrows (up: increased; down: decreased) following “apoptotic,” “proliferation,” “maturation,” and “RTE.” Mechanically, SRSF1 directly binds and regulates the splicing of Irf7 and Il27ra pre-mRNA. In SRSF1-deficient thymocytes, the aberrant splicing of Irf7 and Il27ra mRNAs results in the accelerated degradation of Irf7 transcripts and NMD of Il27ra transcripts, respectively. The protein levels of IRF7 and IL-27Rα were severely decreased (indicate with the magenta arrows), which compromised the expression of the type I interferon–related genes. In addition, SRSF1 deletion also perturbs the expression of Foxo1, Il7ra, Myc, Runx3, Smad7, Bax, and Bcl2l11, contributing to the aberrant apoptosis and proliferation of thymocytes. The black arrows following genes in the boxes indicate the down-regulated expression or up-regulated expression, respectively. The little red balls in the isoforms resulted from ectopic splicing show the generation of PTC.
