Fig. 6. In vivo therapeutic efficacy of CSSP NPs against heterotopic 4T1 tumors.
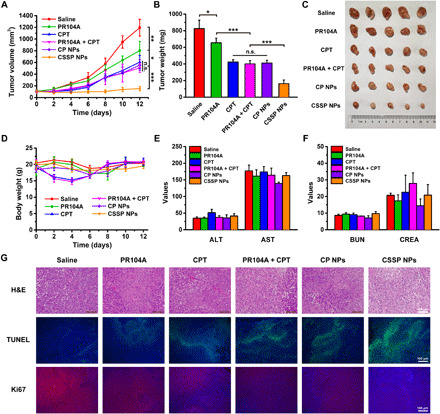
(A) The tumor growth profiles, (B) tumor weights, and (C) tumor photos after various treatments (5.7 mg kg−1, PR104A equivalent; 4 mg kg−1, CPT equivalent) (n = 5). (D) Body weight changes of mice after administration of different formulations (n = 5). (E) Hepatic and (F) renal function parameters of mice after treatments (n = 3). ALT (unit per liter), alanine aminotransferase; AST (unit per liter), aspartate aminotransferase; BUN (millimolar), blood urea nitrogen; CREA (micromolar), creatinine. (G) Hematoxylin and eosin (H&E), terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick end labeling (TUNEL), and Ki67 staining of tumors after mice receiving different treatments. Statistical significance: *P < 0.05, **P < 0.01, and ***P < 0.001. Photo credit: Dongyang Zhao, Department of Pharmaceutics, Wuya College of Innovation, Shenyang Pharmaceutical University, Shenyang 110016, China.
