Abstract
Background:
In recent years, immune checkpoint inhibitors (ICIs) including atezolizumab, durvalumab, pembrolizumab, and nivolumab have reported their efficacy and safety profile in patients with extensive-stage small cell lung cancer (ES-SCLC). However, given the diverse efficacy and inconsistent safety among the ICIs, with the absence of head-to-head researches designed to evaluate the efficacy among them, it might bring with confusion on selection in clinical practice.
Objectives:
The present systematic review and network meta-analysis was performed to conduct indirect comparisons on efficacy and safety profile among ICIs, including atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with ES-SCLC.
Design:
Several databases were retrieved with established criteria until June 20, 2020, with the main MeSH Terms and their similarities. Hazard ratios of overall survival (OS) and progression-free survival (PFS), odds ratios (ORs) of disease control rate (DCR), objective response rate (ORR), and adverse events (AEs) were compared indirectly with network meta-analysis.
Data sources:
Medline, Cochrane library, and Embase.
Eligibility criteria:
Prospective, randomized, controlled clinical studies, which reported PFS, OS, and AEs.
Data extraction and synthesis:
Clinical characteristics were extracted by the 2 authors independently. Comparisons of HRs were calculated for PFS and OS by random effect model. ORR, DCR, and AEs were presented with ORs. Based on surface under the cumulative ranking curve, and forest plots, efficacy and safety of the treatments were ranked, with predicted histogram described.
Results:
In total, there were 4 studies including 1547 patients who met the eligibility criteria and enrolled. For indirect comparisons, no significant difference on PFS was observed between atezolizumab and durvalumab (HR 0.96, 95% CI, 0.72–1.29), or between atezolizumab and pembrolizumab (HR 1.05, 95% CI, 0.78–1.43), or between atezolizumab and nivolumab (HR 1.18, 95% CI, 0.79–1.79), or between durvalumab and pembrolizumab (HR 1.10, 95% CI, 0.84–1.43). or between durvalumab and nivolumab (HR 1.23, 95% CI, 0.83–1.82), or between pembrolizumab and nivolumab (HR 1.12, 95% CI, 0.76–1.66), nor significant difference on OS observed between atezolizumab and durvalumab (HR 0.93, 95% CI, 0.67–1.30), or between atezolizumab and pembrolizumab (HR 0.88, 95% CI, 0.62–1.24), or between atezolizumab and nivolumab (HR 1.04, 95% CI, 0.66–1.66), or between durvalumab and pembrolizumab (HR 0.94, 95% CI, 0.70–1.25), or between durvalumab and nivolumab (HR 1.12, 95% CI, 0.73–1.71), or between pembrolizumab and nivolumab (HR 1.19, 95% CI, 0.77–1.84). However, durvalumab was shown statistical superiority on ORR when compared with atezolizumab (HR 0.79, 95% CI, 0.64–0.98), also with significantly higher risk on immune-related AEs when compared with atezolizumab (OR 0.22, 95% CI, 0.10–0.50), and pembrolizumab (OR 3.12, 95% CI, 1.27–7.64).
Conclusions:
Results of the study revealed that there was no statistical difference on PFS or OS among agents of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with ES-SCLC. However, durvalumab was shown superiority on ORR when compared with atezolizumab, also with significantly higher risk on immune-related AEs.
Keywords: atezolizumab, durvalumab, extensive-stage small cell lung cancer, nivolumab, pembrolizumab
1. Introduction
Small cell lung cancer (SCLC) is characterized by rapid progress, high growth fraction, and early development of widespread metastases, which accounts for approximately 15% to 20% among lung cancer patients.[1,2] Patients with SCLC have always been diagnosed with metastatic disease at first administration. Approximately two-third patients presented with extensive disease.[3] SCLC was highly sensitive to radiotherapy and cell toxicity chemotherapy. However, a majority of patients finally died of recurrent and progressed disease.[4,5] In patients with extensive-stage disease, systemic therapy has been deemed as standard treatment, which significantly palliated symptoms and prolonged survival in most patients. However, long-term survival for patients with extensive-stage disease is still rare.[5] The median overall survival time of patients with extensive-stage SCLC (ES-SCLC) was less than 1 year.
In last decades, etoposide plus platinum has been recommended as a standard treatment option for patients with ES-SCLC, with a preference for carboplatin over cisplatin owing to its equivalent efficacy and more tolerable toxicity profile. Recently, the standard recommendation has been changed because of the publication of a randomized phase III trial (IMpower133), which demonstrates improved survival time (including progression-free survival [PFS] and OS) with the addition of atezolizumab, a PD-L1targeted immune checkpoint inhibitor, to etoposide and cisplatin or carboplatin (EP or EC).[6] In this study, standard EP or EC was compared to the same regimen plus atezolizumab as first-line treatment, followed by maintenance of atezolizumab or placebo in patients with ES-SCLC. The mOS was significantly longer with the addition of atezolizumab (12.3 months (95% CI, 10.8–15.9) vs. 10.3 months (95% CI, 9.3–11.3)) compared with placebo.[6] Subsequently, another PD-L1 targeted immune checkpoint inhibitor durvalumab also reported its positive results on survival time.[7] It was revealed that durvalumab plus EP or EC was associated with a remarkable improvement on OS, with a HR of 0.73 (95% CI, 0.59–0.91; P = .0047). Median OS was 13.0 months (95% CI, 11.5–14.8) in durvalumab plus EP or EC regimen versus 10.3 months in the EP or EC group, with 34% versus 25% of patients alive at 18 months. In addition, immune checkpoint inhibitors including pembrolizumab, and nivolumab also released their clinical data on the most recent ASCO meetings.[8,9]
Given the diverse efficacy and inconsistent safety profile among the immune checkpoint inhibitors, with the absence of head-to-head researches conducted to evaluate the efficacy among them, it might bring with confusion on selection in clinical practice. Based on that, we designed the systematic review, and subsequently performed the present network meta-analysis, aiming to provide a comprehensive conclusion of indirect comparisons on efficacy and safety profile of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with ES-SCLC.
2. Material and methods
2.1. Literature search strategy
Databases including Medline, Cochrane library, and Embase were retrieved (Chen) with the deadline up to June 20, 2020, with the main MeSH Terms, including “Atezolizumab,” “Durvalumab,” “Pembrolizumab,” “Nivolumab,” “small cell lung cancer” and their similarities. Specifically, available terms used in the present study were listed as: “small-cell lung cancer” OR “small cell lung cancer” OR “small cell lung carcinoma” OR “small-cell lung carcinoma” OR “SCLC,” “extensive,” “first line” OR “first-line,” “nivolumab” OR “pembrolizumab” OR “atezolizumab” OR “durvalumab” OR “PD-1 inhibitor” OR “anti-PD-1” OR “anti PD-1” OR “PD-L1 inhibitor” OR “anti-PD-L1” OR “anti PD-L1” and “trial” OR “study” OR “clinical” OR “randomized” OR “randomized” OR “randomly.” The comprehensive retrieve procedure was limited to randomized, prospective, controlled clinical studies, including fully published researches and meeting abstracts belong to American Society of Clinical Oncology (ASCO) meeting, European Society for Medical Oncology (ESMO) congress, World Conference on Lung Cancer (WCLC), and American Association of Cancer Research (AACR). The present network meta-analysis was conducted in compliance with the criteria of the Cochrane handbook for systematic reviews of interventions, with all outcomes reported according to the statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).[10]
2.2. Inclusion and exclusion criteria
Inclusion criteria: prospective, randomized, phase III or II, controlled clinical studies related to Atezolizumab, Durvalumab, Pembrolizumab, or Nivolumab as first-line treatment in patients diagnosed with ES-SCLC. Eligible participants were randomly assigned to receive one of the agents, including atezolizumab, durvalumab, pembrolizumab, or nivolumab in the group experiment, or placebo treatment in the group control; All required outcomes including PFS, OS, and AEs should be reported. Accordingly, the following exclusion criteria were listed as: Retrospective or single-arm researches. Patients diagnosed with limited-stage SCLC or treatments as further-line options. Any review or systematic reviews, correspondence, case studies or comments. For repeated published researches, or update reports for same studies, the most updating data was adopted. In English language.
2.3. Data extraction and outcomes
Two authors (JC and JW) extracted relevant data independently, and a third author (HX) was consulted to resolve discrepancies when necessary. Viewpoints held by 2 investigators would be the final decision. Essential clinical characteristics extracted from the enrolled studies including: names of the researches, numbers of patients included, median age, gender, racial distribution, ECOG performance status, smoking history, brain metastasis status, liver metastasis status, and median sum of longest diameter of target lesions. During the data exaction of included studies, we checked and reviewed the clinical trials registries on www.clinicaltrials.gov. The primary outcomes evaluated in the present network meta-analysis were PFS (randomization to progression of any causes or death regardless of any causes) and OS (randomization to death regardless of any causes). Secondary endpoints items consisted of ORR (patients evaluated as complete response (CR) or partial response (PR) according to the criteria of RECIST version 1.1), disease control rate (DCR) (patients evaluated as CR or PR or stable disease), AEs lead to treatment discontinuation, AEs at high grade (≥ grade 3), fatal adverse events (FAEs), immune-related AEs, and specific AEs including hypothyroidism, hyperthyroidism, rash, pneumonitis, hepatitis, and colitis.
2.4. Quality assessment of included studies
Cochrane Collaboration's tool for assessing risk of bias of RCTs was adopted for quality evaluation in the present study by the 2 reviewers (JC and JW). The following items were deemed as necessary criteria for assessment: allocation concealment, random sequence generation, binding of outcome assessments, binding of participants and personnel, incomplete outcome data, selective reporting and other bias, with results of which presented as “risk of bias graph" and “risk of bias summary.”
2.5. Statistical analysis
All comparisons including direct and indirect ones in the present study were analyzed in software STATA version 13.0 (StataCorp, College Station, TX). The 5 interventions including atezolizumab, durvalumab, pembrolizumab, nivolumab, and placebo were divided into all possible combinations by 2 intervention tests. Based on the absence of a closed loop for the indirect comparison, consistency or inconsistency test was exempted. Comparisons of HRs, as well as variance estimates, were calculated from the reported CIs for PFS and OS by random effect model. ORR, DCR, AEs lead to treatment discontinuation, AEs at high grade (≥ grade 3), FAEs, immune-related AEs, and specific AEs were presented with ORs. In terms of indirect comparison among Atezolizumab, Durvalumab, Pembrolizumab, and Nivolumab, network meta-analysis methods (STATA network) were adopted for the final analysis. Eventually, based on surface under the cumulative ranking (SUCRA) curve, and forest plots, efficacy and safety of the treatments were ranked, with predicted histogram described. In addition, the software RevMan version 5.3 (Cochrane Corp, UK) was used for the description of PRISMA flow diagram, risk of bias summary, and risk of bias graph. The publication bias of the literature was evaluated with funnel diagrams.
3. Results
3.1. Literature review
With an integrative retrieve among databases including Embase, Medline, and Cochrane Library, a total of 1954 potential literatures were initially enrolled. Eight hundred sixteen publications were removed because of duplications. Twenty-three researches were further excluded with the property of prospective, randomized, placebo controlled characteristic for satisfactory researches. After full text carefully reviewed, 30 papers were excluded with the reasons listed in appendix, Supplemental Digital Content, remaining 4 clinical trials considered eligible for the final analysis.[6–9] A flow diagram that detailed the selection of the included studies was presented in Figure 1. All the included researches were randomized, control designed, and fit the criteria and the requirements of the present study.
Figure 1.
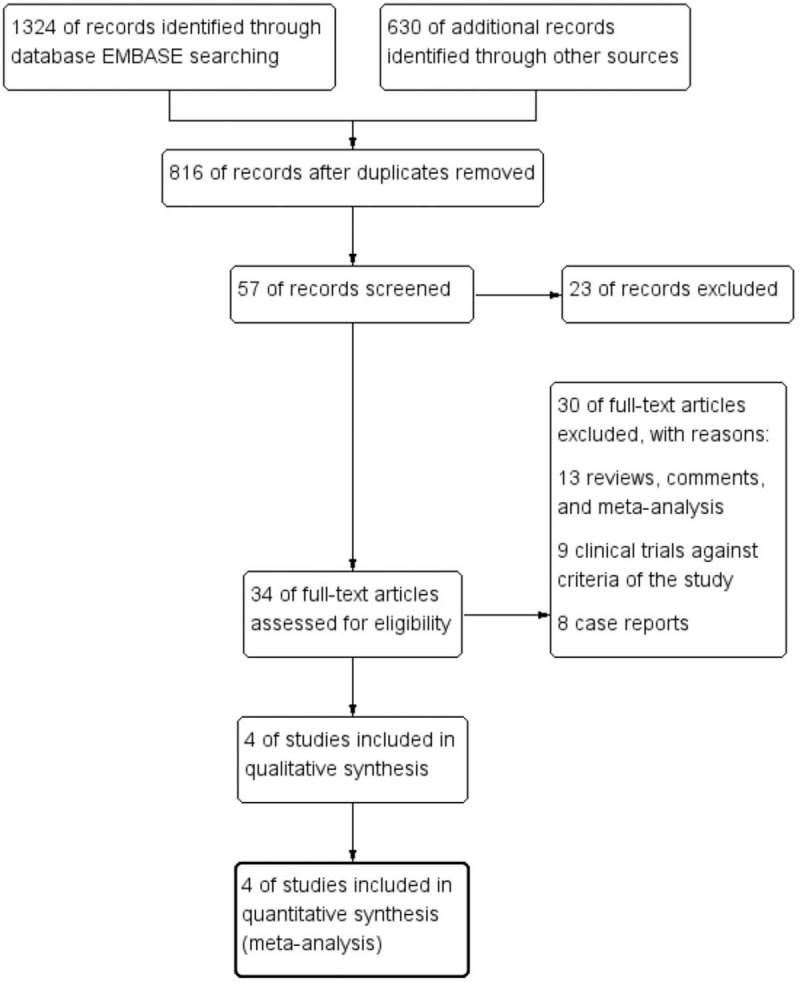
Study selection procedure with PRISMA flow diagram.
3.2. Quality assessment of the included studies
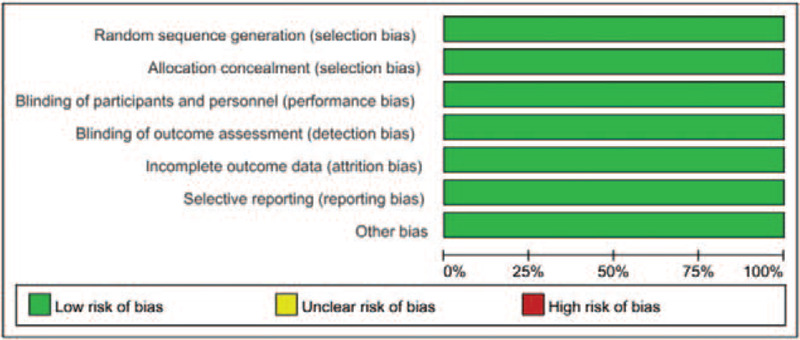
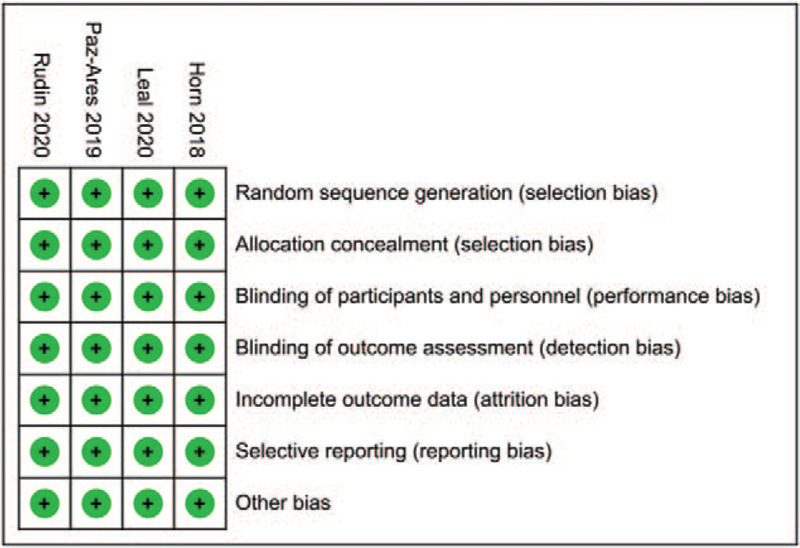
With the performance of quality assessment according to the criteria of Cochrane Collaboration's tool, we detected that all of the included researches in the present study satisfied the criteria items including allocation concealment, random sequence generation, binding of outcome assessments, and binding of participants and personnel, with results presented in Figures 2 and 3.
Figure 2.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Figure 3.
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages among all the included studies.
3.3. Study design and the population characteristics
A total of 4 studies (marked as Impower133,[6] CASPIAN,[7] KEYNOTE604,[8] and ECOG-ACRIN EA5161[9]) including 1547 patients were considered available in the present network meta-analysis. All of the patients included in the present study were histologically or cytologically confirmed ES-SCLC. To be specific, the IMpower133 trial, patients were randomly assigned to receive intravenously with platinum-etoposide regimens combined with either atezolizumab at a dose of 1200 mg or placebo. The induction treatment was followed by a maintenance treatment during which patients received either atezolizumab or placebo until the occurrence of unacceptable toxic effects or disease progression.[6] In terms of trial CASPIAN, patients were assigned to receive durvalumab plus etoposide-cisplatin (EP) or etoposide-carboplatin (EC) regimen. Patients received up to 4 cycles of chemotherapy plus durvalumab 1500 mg followed by maintenance durvalumab 1500 mg every 4 weeks or placebo.[7] In the trial KEYNOTE604, patients were randomly assigned 1:1 to receive 4 cycles of etoposide and platinum, followed by pembrolizumab at a dose of 200 mg once every 3 weeks or placebo for up to 35 cycles.[8] While in ECOG-ACRIN EA5161, patients were also randomly assigned 1:1 to nivolumab at a dose of 360 mg once every 3 weeks plus 4 cycles of etoposide/carboplatin and platinum or etoposide/carboplatin and platinum alone.[9] The baseline clinic-pathological characteristics in all the included researches were presented in Table 1.
Table 1.
Baseline characteristics of the studies included in the present meta-analysis (ITT population).
| IMpower133 | CASPIAN | KEYNOTE604 | EA5161 | |||||
| Atez | PLA | Durv | PLA | Pemb | PLA | Nivo | PLA | |
| Number | N = 201 (%) | N = 202 (%) | N = 268 (%) | N = 269 (%) | N = 228 (%) | N = 225 (%) | N = 80 (%) | N = 80 (%) |
| Median age (range) | 64 (28–90) | 64 (26–87) | 62 (58–68) | 63 (57–68) | 64 (24–81) | 65 (37–83) | 65 | 65 |
| Gender | ||||||||
| Male | 129 (64.2) | 132 (65.3) | 190 (71) | 184 (68) | 152 (66.7) | 142 (63.1) | 35 (44) | 36 (45) |
| Female | 72 (35.8) | 70 (34.7) | 78 (29) | 85 (32) | 76 (33.3) | 83 (36.9) | 45 (56) | 44 (55) |
| Smoking history | ||||||||
| Never | 9 (4.5) | 3 (1.5) | 22 (8) | 15 (6) | 8 (3.5) | 8 (3.6) | NR | NR |
| Current | 74 (36.8) | 75 (37.1) | 120 (45) | 126 (47) | 148 (64.9) | 133 (59.1) | NR | NR |
| Previous | 118 (58.7) | 124 (61.4) | 126 (47) | 128 (48) | 72 (31.6) | 84 (37.7) | NR | NR |
| ECOG PS | ||||||||
| 0 | 73 (36.3) | 67 (33.2) | 99 (37) | 90 (33) | 60 (26.3) | 56 (24.9) | 23 (49) | 24 (51) |
| 1 | 128 (63.7) | 135 (66.8) | 169 (63) | 179 (67) | 168 (73.7) | 169 (75.1) | 57 (51) | 56 (49) |
| Race | ||||||||
| Asian | 40 (19.9) | 40 (19.8) | 36 (13) | 42 (16) | 52 (22.8) | 32 (14.2) | NR | NR |
| Other | 161 (80.1) | 162 (80.2) | 232 (87) | 227 (84) | 176 (77.2) | 193 (85.8) | NR | NR |
| Brain metastasis | ||||||||
| Yes | 17 (8.5) | 18 (8.9) | 28 (10) | 27 (10) | 33 (14.5) | 22 (9.8) | NR | NR |
| No | 184 (91.5) | 184 (91.1) | 240 (90) | 242 (90) | 195 (85.5) | 203 (90.2) | NR | NR |
| Liver metastasis | ||||||||
| Yes | 77 (38.3) | 72 (35.6) | 108 (40) | 104 (39) | 95 (41.7) | 92 (40.9) | NR | NR |
| No | 124 (61.7) | 130 (64.4) | 160 (60) | 165 (61) | 133 (58.3) | 133 (59.1) | NR | NR |
| Median sum of longest diameter of target lesions, mm (range) | 113.0 (12.0–325.0) | 105.5 (15.0–353.0) | NR | NR | 134.8 (24.4–431.7) | 126.6 (20.8–408.8) | NR | NR |
The model of the comparison developed with network was presented in Figure 4. All the drugs including atezolizumab, durvalumab, pembrolizumab, and nivolumab were compared separately with placebo. Figure 5 showed the contribution plot of the included literatures with network.
Figure 4.
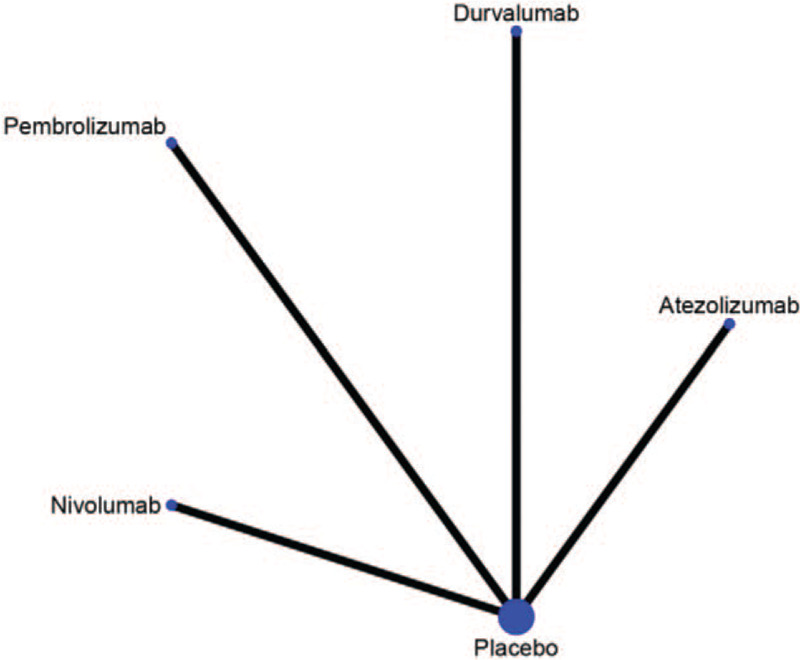
Network of the comparisons.
Figure 5.
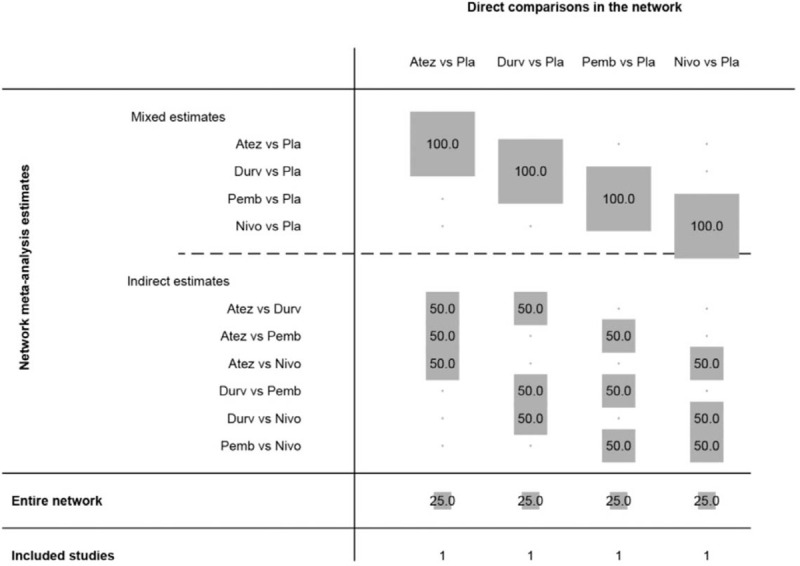
Contribution plot of the included literatures.
3.4. Efficacy
For PFS in the direct and indirect comparisons, all the drugs showed statistical benefit when compared with placebo (HR for atezolizumab 0.77, 95% CI, 0.61–0.97; HR for durvalumab0.80, 95% CI, 0.66–0.97; HR for pembrolizumab 0.73, 95% CI, 0.60–0.89; HR for nivolumab 0.65, 95% CI, 0.46–0.91). However, no significant difference was observed in the indirect comparison between atezolizumab and durvalumab (HR 0.96, 95% CI, 0.72–1.29), nor the one between atezolizumab and pembrolizumab (HR 1.05, 95% CI, 0.78–1.43), or between atezolizumab and nivolumab (HR 1.18, 95% CI, 0.79–1.79), or between durvalumab and pembrolizumab (HR 1.10, 95% CI, 0.84–1.43). or between durvalumab and nivolumab (HR 1.23, 95% CI, 0.83–1.82), or between pembrolizumab and nivolumab (HR 1.12, 95% CI, 0.76–1.66). All the results of comparisons for PFS were presented in Figure 6A and C.
Figure 6.
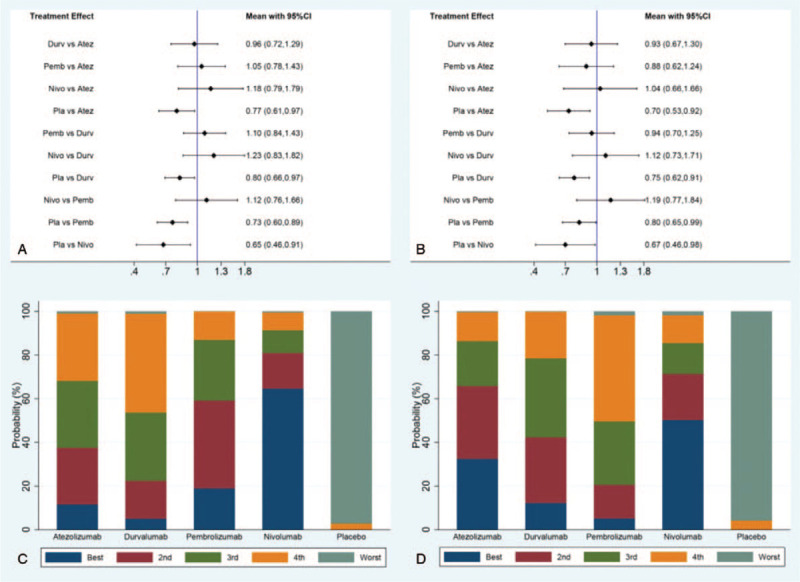
Direct and indirect comparisons for PFS (A/C) and OS (B/D) among Atez (atezolizumab), Durv (durvalumab), Pemb (pembrolizumab), Nivo (nivolumab), and Pla (placebo).
For OS in the direct and indirect comparison, all the drugs showed statistical benefit when compared with placebo (HR for atezolizumab 0.70, 95% CI, 0.53–0.92; HR for durvalumab 0.75, 95% CI, 0.62–0.91; HR for pembrolizumab 0.80, 95% CI, 0.65–0.99; HR for nivolumab 0.67, 95% CI, 0.46–0.98). However, no significant difference was observed in the indirect comparison between atezolizumab and durvalumab (HR 0.93, 95% CI, 0.67–1.30), nor the one between atezolizumab and pembrolizumab (HR 0.88, 95% CI, 0.62–1.24), or between atezolizumab and nivolumab (HR 1.04, 95% CI, 0.66–1.66), or between durvalumab and pembrolizumab (HR 0.94, 95% CI, 0.70–1.25), or between durvalumab and nivolumab (HR 1.12, 95% CI, 0.73–1.71), or between pembrolizumab and nivolumab (HR 1.19, 95% CI, 0.77–1.84). All the results of comparisons for OS were presented in Figure 6B and D.
In the indirect analysis of short-term efficacy including ORR and DCR, the only statistical difference among the 4 agents was presented between atezolizumab and durvalumab on ORR. The ORR of durvalumab was shown superiority compared with atezolizumab (HR 0.79, 95% CI, 0.64–0.98). All the results of comparison for ORR and DCR were presented in Figure 7.
Figure 7.
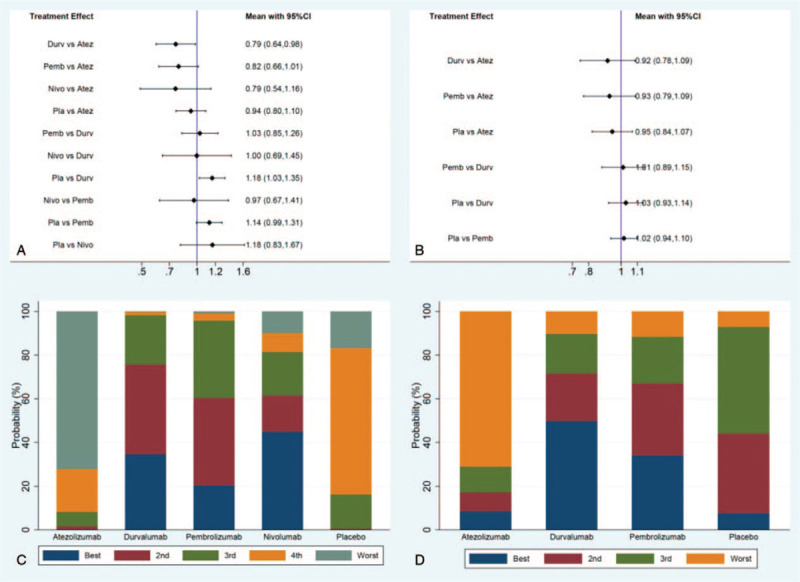
Direct and indirect comparisons for ORR (A/C) and DCR (B/D) among Atez (atezolizumab), Durv (durvalumab), Pemb (pembrolizumab), Nivo (nivolumab), and Pla (placebo).
3.5. Safety
For the safety analysis, direct and indirect comparisons of AEs lead to treatment discontinuation, AEs at high grade (≥ grade 3), FAEs, and immune-related AEs were conducted among drugs including atezolizumab, durvalumab, pembrolizumab, and placebo. As a result, durvalumab was shown statistically significantly higher immune-related AEs when compared with atezolizumab (OR 0.22, 95% CI, 0.10–0.50), and pembrolizumab (OR 3.12, 95% CI, 1.27–7.64). Otherwise, there is no statistical difference observed between the other direct or indirect comparisons, results of which were presented in Figure 8.
Figure 8.
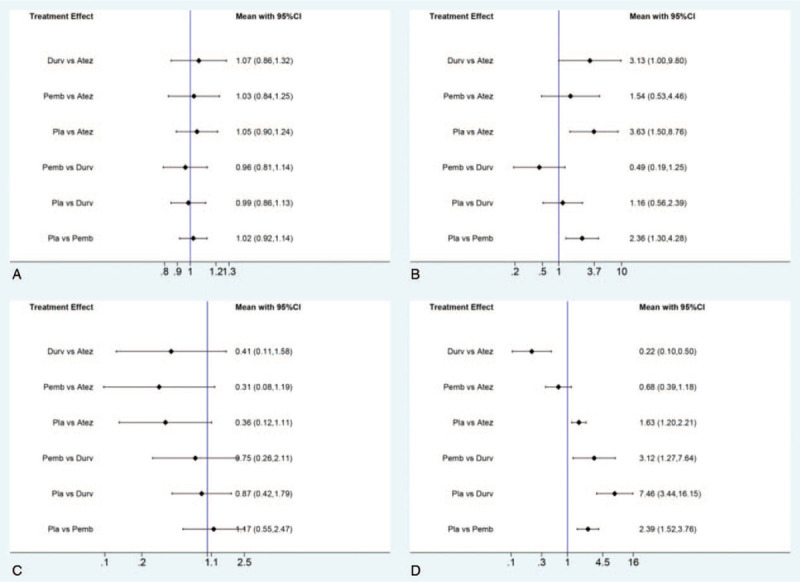
Direct and indirect comparisons for AEs lead to treatment discontinuation (A), AEs at high grade (≥ grade 3) (B), FAEs (C), and immune-related AEs (D).
In addition, direct and indirect comparisons of specific AEs including hypothyroidism, hyperthyroidism, rash, pneumonitis, hepatitis, and colitis were also conducted among atezolizumab, durvalumab, pembrolizumab, and placebo. As a result, durvalumab was shown a statistically higher risk of hyperthyroidism compared with placebo (OR 29.09, 95% CI, 1.74–485.09). However, atezolizumab might be revealed a higher risk of rash compared with placebo (OR 1.83, 95% CI, 1.10–3.04). Otherwise, there was no significant difference observed on any other specific AEs among the comparison of atezolizumab, durvalumab, pembrolizumab, and placebo, with results of which presented in Figure 9.
Figure 9.
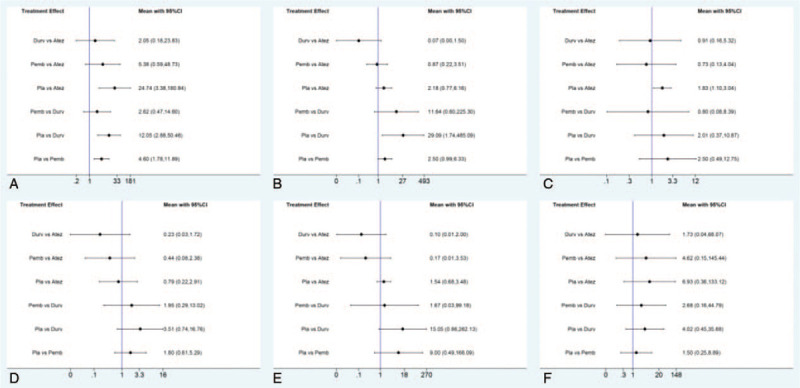
Direct and indirect comparisons for specific AEs including hypothyroidism (A), hyperthyroidism (B), rash (C), pneumonitis (D), hepatitis (E), and colitis (F).
3.6. Publication bias
Funnel plot of network meta-analysis did not reveal significant publication bias in the present study (Fig. 10).
Figure 10.
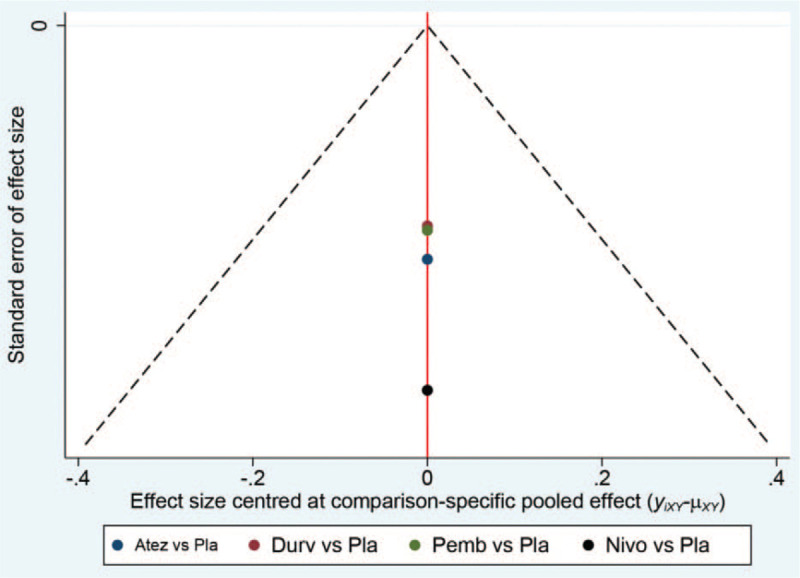
Funnel plot for the detection of publication bias.
4. Discussion
The results of the present network meta-analysis suggested that there was no statistical difference observed in the indirect comparison on PFS or OS among agents of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with ES-SCLC. However, durvalumab was shown superiority on ORR when compared with atezolizumab (HR 0.79, 95% CI, 0.64–0.98), also with significantly higher risk on immune-related AEs when compared with atezolizumab (OR 0.22, 95% CI, 0.10–0.50), and pembrolizumab (OR 3.12, 95% CI, 1.27–7.64). In addition, durvalumab was shown a statistically higher risk of hyperthyroidism compared with placebo (OR 29.09, 95% CI, 1.74–485.09). However, atezolizumab might be suggested a higher risk of rash compared with placebo (OR 1.83, 95% CI, 1.10–3.04).
Attempt of immune checkpoint inhibitors during the treatment of SCLC was started as further-line administration in patients with relapsed ES-SCLC.[11] The trial CheckMate 032 was initially conducted to evaluate nivolumab plus ipilimumab or nivolumab alone in patients with relapsed SCLC. The results revealed ORR were 10% for nivolumab at a dose of 3 mg/kg, 23% for nivolumab at a dose of 1 mg/kg plus ipilimumab at a dose of 3 mg/kg, and 19% for nivolumab at a dose of3 mg/kg plus ipilimumab at a dose of1 mg/kg.[12] Hereafter, another phase III trial (CheckMate 331) also assessed nivolumab versus cell toxicity agents including topotecan or amrubicin in patients with relapsed SCLC, preliminary data of which showed that OS was similar between the 2 groups (HR, 0.86 (95% CI, 0.72–1.04); P = .11).[13] In terms of safety profile, treatment-related FAEs occurred in 2 patients in group nivolumab and 3 patients received chemotherapy.[13] Based on that, the NCCN panel recommended nivolumab or nivolumab plus ipilimumab as subsequent treatment choice for patients who have relapsed less than 6 months after primary or maintenance therapy. In addition, a recent combined analysis of 2 studies (KEYNOTE-028 and KEYNOTE-158) was conducted to evaluate the efficacy and safety of pembrolizumab in patients with relapsed SCLC, results of which reported an ORR of 19.3% and a median OS of 7.7 month. Both ORR and OS were significantly higher in patients with positive PD-L1 expression.[14] Hence, the NCCN panel also recommended pembrolizumab as one of further-line treatment options in patients with SCLC regardless of PD-L1 expression status.
The recommendation of NCCN SCLC panel for first-line treatment of ES-SCLC was updated because of the landmark results of IMpower133. With the positive and encouraging extension on PFS and OS, NCCN SCLC Panel recommended atezolizumab plus EP or EC regimens as the preferred first-line systemic therapy followed by maintenance atezolizumab in patients with ES-SCLC.[6] Thereafter, reported results of a randomized trial CASPIAN also revealed that first-line durvalumab plus platinum-etoposide significantly improved OS and PFS compared with standard EP or EC regimens in patients with ES-SCLC. Other than the encouraging results of PD-L1 inhibitors, the attempt of PD-1 inhibitor encountered difficulties during the first-line treatment of ES-SCLC. The randomized, trial KEYNOTE-604 compared pembrolizumab plus EP with placebo plus EP as first-line treatment for patients with ES-SCLC, results of which revealed that the addition of pembrolizumab significantly improved PFS (HR, 0.75, P = .0023). Although the HR of OS favored pembrolizumab plus EP, the prespecified significance threshold was narrowly missed (HR, 0.80; P = .0164).[15] However, the OS curves in KEYNOTE604 diverged in favor of pembrolizumab starting at 5 months, with the separation maintained over time on the follow-up. OS rates by 12 months and 24 months were 45.1%, and 22.5% in group pembrolizumab versus 39.6%, and 11.2% in group placebo, respectively. Restricted mean survival time analysis of PFS and OS also supported the advantage outcomes in favor of the addition of pembrolizumab over the whole period of follow-up. In terms of nivolumab, the phase II trial presented encouraging results on PFS and OS, the subsequent phase III research is still anticipated.
Although the efficacy of immune checkpoint inhibitors has been proved promising, immune-mediated adverse events caused by which should be paid enough attention to. Immune-mediated AEs are discrete toxicities, caused by abnormal activation of systematic immune system, which usually affect multiple organs. Meta-analysis revealed the incidence of immune-mediated AEs caused by anti-PD-1/PD-L1 agents might be approximately 30%, with less than 20% individual suffering severe AEs (≥ grade 3).[16–18] Therefore, health care providers should be aware of the spectrum of potential immune-mediated AEs including hypothyroidism, hyperthyroidism, rash, pneumonitis, hepatitis, colitis, and so on. At the meeting of WCLC in 2019, a research team reported a retrospective study that investigated the relationship between the immune-mediated adverse events and efficacy, results of which suggested that patients with immune-mediated adverse events might result with a better ORR, PFS, and OS.[19] The outcomes of the retrospective research revealed that ORR was significantly higher in patients with immune-mediated AEs (26.3% versus 3.3%, P < .001), as well as statistical advantages on PFS (4.1 months versus 1.3 months, HR = 0.30, 95% CI, 0.20–0.43, P < .001), and OS (14.1 months versus 2.9 months, HR = 0.32, 95% CI, 0.21–0.48, P < .001) compared with patients without any immune-mediated AEs.[19] The similar result was partially proved in the present study. Indirect comparison of the present study revealed that durvalumab was shown a superiority on ORR when compared with atezolizumab (HR 0.79, 95% CI, 0.64–0.98), however, with significantly higher risk on immune-related AEs when compared with atezolizumab (OR 0.22, 95% CI, 0.10–0.50), and pembrolizumab (OR 3.12, 95% CI, 1.27–7.64). Even so, immune-mediated adverse events should also be brought to the forefront in clinical practice.
It should be acknowledged that some limitations were existed in the present network meta-analysis, the most obvious one was the heterogeneity, which might be caused by the diversity racial population. Most of the included trials recruited patients in non-Asian population. It remains controversial that whether the conclusion of the pooled analysis could represent the real-world status of patients with ES-SCLC in Asian, especially in China. In addition, the trial ECOG-ACRIN EA5161 was a phase II study, with limited sample. A phase III randomized, controlled study with larger sample is needed. Hence, the explanation on efficacy and safety of nivolumab should be cautiously approached. Finally, available data was not obtained directly from the individuals in the including research, which would have brought with potential bias.
In summary, the present network meta-analysis revealed that there was no statistical difference observed in the indirect comparison of PFS or OS among agents of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with extensive-stage SCLC. Besides, durvalumab was shown superiority on ORR when compared with atezolizumab, however, with significantly higher risk on immune-related AEs when compared with atezolizumab and pembrolizumab.
Acknowledgments
The authors thank all the patients enrolled in the clinical trials included in the present study.
Author contributions
Conceptualization: jianxin chen.
Data curation: jianxin chen, hui xu.
Formal analysis: jianxin chen, junhui wang.
Methodology: jianxin chen, junhui wang.
Supervision: junhui wang.
Validation: hui xu.
Writing – original draft: jianxin chen.
Writing – review & editing: hui xu.
Supplementary Material
Footnotes
Abbreviations: AACR = American Association of Cancer Research, AE = adverse event, ASCO = American Society of Clinical Oncology, CR = complete response, DCR = disease control rate, EC = etoposide-carboplatin, EP = etoposide-cisplatin, ESMO = European Society for Medical Oncology, ES-SCLC = extensive-stage small cell lung cancer, FAE = fatal adverse event, OR = odds ratio, ORR = objective response rate, OS = overall survival, PFS = progression-free survival, PR = partial response, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, WCLC = World Conference on Lung Cancer.
How to cite this article: Chen J, Wang J, Xu H. Comparison of atezolizumab, durvalumab, pembrolizumab, and nivolumab as first-line treatment in patients with extensive-stage small cell lung cancer: a systematic review and network meta-analysis. Medicine. 2021;100:15(e25180).
HX and JW contributed equally to the present study.
The present meta-analysis was not funded.
The ethical approval was waived, because of the nature of a meta-analysis for the present study. This article does not contain any studies with animals performed by any of the authors.
Informed consent was not available as it was a systematic review.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Supplemental digital content is available for this article.
Atez = Atezolizumab, Durz = Durvalumab, ECOG PS = eastern cooperative oncology group performance status, Nivo = Nivolumab, NR = not reported, Pemb = Pembrolizumab, PLA = placebo.
References
- [1].Welter S, Aigner C, Roesel C. The role of surgery in high grade neuroendocrine tumours of the lung. J Thorac Dis 2017;9:S1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fasano M, Della Corte CM, Papaccio F, et al. Pulmonary large-cell neuroendocrine carcinoma: from epidemiology to therapy. J Thorac Oncol 2015;10:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals for the revision of the clinical and pathologic staging of small cell lung cancer in the forthcoming eighth edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:300–11. [DOI] [PubMed] [Google Scholar]
- [4].Jett JR, Schild SE, Kesler KA, et al. Treatment of small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e400S–19S. [DOI] [PubMed] [Google Scholar]
- [5].Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J 2010;35:202–15. [DOI] [PubMed] [Google Scholar]
- [6].Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. [DOI] [PubMed] [Google Scholar]
- [7].Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet 2019;394:1929–39. [DOI] [PubMed] [Google Scholar]
- [8].Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 2020;38:2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leal T, Wang Y, Dowlati A, et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOGACRIN EA5161. J Clin Oncol 2020;38: suppl 15: 9000. [Google Scholar]
- [10].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [11].Horn L, Reck M, Spigel DR. The future of immunotherapy in the treatment of small cell lung cancer. Oncologist 2016;21:910–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. [DOI] [PubMed] [Google Scholar]
- [13].Reck M, Vicente D, Ciuleanu T, et al. Efficacy and safety of nivolumab (nivo) monotherapy versus chemotherapy (chemo) in recurrent small cell lung cancer (SCLC): results from CheckMate 331. Ann Oncol 2018;29:x32–3. [Google Scholar]
- [14].Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 2020;15:618–27. [DOI] [PubMed] [Google Scholar]
- [15].Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, Phase III KEYNOTE-604 study. J Clin Oncol 2020;38:2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Linardou H, Gogas H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med 2016;4:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maughan BL, Bailey E, Gill DM, et al. Incidence of immune-related adverse events with program death receptor-1- and program death receptor-1 ligand-directed therapies in genitourinary cancers. Front Oncol 2017;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kumar V, Chaudhary N, Garg M, et al. Corrigendum: current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ricciuti B, Naqash AR, Henick B, et al. OA03.07 immune-related adverse events and clinical outcome to anti PD-1 axis inhibition in SCLC: a multicenter retrospective analysis. J Thorac Oncol 2019;14:S213–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


