Abstract
Since 2018, 2 chimeric antigen receptor (CAR) T-cell therapies received approval from the European Medicine Agency, with list prices around 320 000 Euro (€) (EUR) per treatment. These high prices raise concerns for patient access and the sustainability of healthcare systems. We aimed to estimate the costs and budget impact associated with CAR T-cell therapies for current and future indications in hematological cancers from 2019 to 2029. We focused on the former France, Germany, Spain, Italy and the United Kingdom (EU-5) and the Netherlands. We conducted a review of list prices, health technology assessment reports, budget impact analysis dossiers, and published cost-effectiveness analyses. We forecasted the 10-year health expenditures on CAR T-cells for several hematological cancers in selected European Union countries. Nine cost-effectiveness studies were identified and list prices for CAR T-cell therapies ranged between 307 200 EUR and 350 000 EUR. Estimated additional costs for pre- and post-treatment were 50 359 EUR per patient, whereas the incremental costs of CAR T-cell therapy (when compared with care as usual) ranged between 276 086 EUR and 328 727 EUR. We estimated market entry of CAR T-cell therapies for chronic mantle cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia, multiple myeloma, and acute myeloid leukemia in 2021, 2022, 2022, 2022, and 2025, respectively. Cumulative expenditure estimates for existing and future indications from 2019 to 2029 were on average 28.5 billion EUR, 32.8 billion EUR, and 28.9 billion EUR when considering CAR T-cell therapy costs only, CAR T-cell therapy costs including pre- and post-treatment, and incremental CAR T-cell therapy costs, respectively. CAR T-cell therapies seem to be promising treatment options for hematological cancers but the financial burden on healthcare systems in the former EU-5 and the Netherlands will contribute to a substantial rise in healthcare expenditure in the field of hematology.
Introduction
It took almost 40 years from the time chimeric antigen receptor (CAR) T-cell therapy was first described in the 1980s to the approval of tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta) by both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in 2017 and 2018, respectively.1 Thus far, the EMA approved tisagenlecleucel for the treatment of pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that are refractory, in relapse post-transplant or in second or later relapse as well as for adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after 2 or more lines of systemic therapy. Axicabtagene ciloleucel is currently approved by the EMA for the treatment of adult patients with relapsed or refractory DLBCL and primary mediastinal large B-cell lymphoma after 2 or more lines of systemic therapy. Both therapies are autologous treatments and second-generation CAR-Ts.
After novel drugs receive central approval by the EMA, each European member state handles its own approval and reimbursement procedure. With list prices of approximately 289 550 Euro (€) (EUR) (373 000 United States dollars [$] [USD]) in the United States and 320 000 EUR in Europe, CAR T-cell therapies belong to the most expensive cancer treatments at the moment. This has consequently raised concerns regarding patient access to these therapies and the financial sustainability of healthcare systems in general. CAR T-cell therapies are expected to bring substantial health benefits but also exposes healthcare systems to very large expenditures. Simultaneously, an increase in trial activity heralds an expansion of CAR T-cell therapies to many more indications in the near future, of which hematological cancers currently play the most significant role.2 Therefore, these therapies may have a considerable incremental budget impact on healthcare expenditures, especially in the field of hematology-oncology. Moreover, the costs associated with these therapies are not limited to acquisition costs alone. Other costs that will have a substantial impact on healthcare expenditures are hospitalization, intensive care unit (ICU) stays, as well as other costs related to the treatment of adverse events (AEs) and laboratory work. Furthermore, patients who live longer will also incur future medical costs unrelated to their condition for which they received CAR T-cell therapy. Conversely, longer survival may also lead to a return to productive work of survivors in remission.
In addition, substitution effects may reduce the financial impact of CAR T-cells such as avoiding the current standard of care treatment and a potential reduction in the numbers of autologous and/or allogeneic stem cell transplantation following treatment.
Overall, the application of CAR T-cell therapies may result in higher overall healthcare spending and opportunity costs—money can only be spent once—leading to a change in the allocation of the available healthcare budget. Without any formal assessment with regards to the financial aspects of these therapies, their costs remain intangible and vague. Even though economic evaluations and budget impact analyses can shed light on the economic burden of new therapies in general, such assessments are not formally required in most countries (in Europe and elsewhere) for drug reimbursement decision making, and therefore, such data are scarce.
The European Hematology Association (EHA) is concerned about the sustainability of the pricing of new oncological treatments, and in particular of CAR T-cell therapy, possibly exposing health systems to very large expenditures. Therefore, the EHA has commissioned the Institute for Medical Technology Assessment (iMTA) to forecast future health expenditures, based on the adoption of CAR T-cell therapies in hematological cancers.
This study aimed to estimate the costs and budget impact associated with CAR T-cell therapies for current and future indications in hematological cancers in Europe from 2019 to 2029. The results of this study can be used by healthcare decision-makers in their budgetary planning as they elucidate the future economic burden of CAR T-cell therapies in several European countries.
Materials and Methods
We followed a 4-stepped approach and focused on 6 European member states: the former EU-5 (ie, Germany, Spain, France, United Kingdom, and Italy) and the Netherlands. First, we conducted a review of list prices, health technology assessment (HTA) reports, budget impact analysis (BIA) dossiers, and published cost-effectiveness analyses (CEA). Second, we identified potential future indications and estimated the eligible patient population for both registered and selected upcoming indications. Third, we validated our findings with international clinical experts in the field of hematology-oncology. Finally, based on the gathered information in the previous steps, we predicted the 10-year health expenditures on CAR T-cells for several hematological cancers in the selected European Union (EU) member states. The forecast entails different cost calculations, namely: (1) costs of CAR T-cell therapies only; (2) costs of CAR T-cell therapies and costs of care, as well as (3) incremental costs associated with the substitution of former therapies by CAR T-cell therapies. All costs are expressed in 2018 Euro.
Review of list prices and cost-effectiveness publications
We retrieved list prices for tisagenlecleucel and axicabtagene ciloleucel from HTA/BIA reports published by national reimbursement authorities. In addition, we searched for published CEA studies to complement potential missing or unpublished data. These publications were searched through Excerpta Medica dataBASE on May 9, 2019, with an update search on April 20, 2020 (see Appendix 1, http://links.lww.com/HS/A130 for the full search strategy). Only economic evaluations for hematological diseases were included.
Identification of future indications and estimation of the eligible patient population
To identify future indications for CAR T-cells, we searched ClinicalTrials.gov for all registered studies on CAR T-cell therapies (search term: “chimeric antigen receptor”) for hematological cancers on May 3, 2019. This search included early phase 1, phase 1, phase 2, phase 3, and phase 4 trials. All studies were ranked according to the indication studied (most to least often studied indication). Through a semi-structured interview, several clinical experts were asked to validate this ranking and to (re)arrange it according to the sequence of expected market entry.
To estimate the eligible patient population for CAR T-cells, we focused on the 2 indications for which CAR T-cells already have market authorization (pediatric ALL [pALL] and DLBCL) and the top 5 potential future indications identified by the clinical experts. The eligible patient population was calculated based on previous population forecasts by using 2 data sources, namely Eurostat and Globocan.3
In the Eurostat forecast, several assumptions were made on the future development for fertility, mortality, and net migration to predict the population of European member states to the year 2080 (based on the population in 2016).4 We assumed a linear trend between the 2016 and 2080 Eurostat data and calculated the yearly population per country of interest. For our purpose, we defined the “disease incident population” by estimating the yearly crude incidence rate (IR) per 100 000 for each disease and country of interest. For pALL and DLBCL, the yearly disease IRs were taken from HTA/BIA reports. For future indications, or in the absence of published data from HTA/BIA reports, we used data from the European Cancer Information System (ECIS).5 Subsequently, the crude IRs were applied to projected population data by Eurostat.4
The online database Global Cancer Incidence, Mortality and Prevalence offers information on projected IRs of different cancer types for the time between 2018 and 2040 for several countries.6 To derive the number of patients for each cancer subtype of interest, we applied proportions based on the literature.3,7–9
Both forecast approaches are depicted in Figure 1.
Figure 1.

Flowchart of forecast approaches. CAR-T = chimeric antigen receptor T cell.
The proportion of “patients eligible for CAR T-cell therapy” per country was calculated based on HTA/BIA reports. Most publications stated the yearly number of incident cases and the total number of patients eligible for CAR T-cell therapy. From these numbers, we calculated the proportion of eligible patients and applied this rate to all incident cases to derive the total yearly number of eligible patients for CAR T-cells per disease and country. The CAR T-cell therapy eligible patient population for all future indications was based on expert opinion.
Validation with clinical experts
Clinical experts in the field of hematology-oncology were asked to validate our intermediate findings via semi-structured interviews. Respondents were asked about their experience with CAR T-cell therapies, possible future hematological indications, resource use during pre-treatment, treatment, and post-treatment with CAR T-cell therapies in their own country, and the plausibility for CAR T-cell therapies to be manufactured within specialized hospitals (point-of-care manufacturing).
Expenditure estimation of CAR T-cell therapies for current and selected future indications
Expenditures were estimated for 3 scenarios. In “Scenario 1,” the CAR T-cell therapy eligible patient population was multiplied with the average list price for the currently approved CAR T-cell therapies in the former EU-5 and the Netherlands. For all new indications, the costs for CAR T-cell therapies were assumed to be similar to the average list price.
For “Scenario 2,” we added costs for pre-treatment, concomitant medication, AEs, and hospitalization (including follow-up) to the price of CAR T-cell therapy. Information on resource use (ie, medication dosage and the number of hospital days) were taken from available HTA/BIA reports or based on expert opinion. Prices for medication, hospitalization (including ICU admission), and AEs were based on costs reported in HTA/BIA reports or the literature.10-16 In case country-specific prices could not be found, the average of available prices was used. Finally, clinical experts were asked to validate these data.
For “Scenario 3,” we calculated the incremental costs of CAR T-cell therapy, that is, the costs of “Scenario 2” minus the costs of care as usual. These incremental costs were derived from the published CEAs identified for this study. Thereafter, we multiplied the eligible patient population with incremental costs of CAR T-cell therapy. Average incremental costs observed in DLBCL were used to estimate incremental costs for future indications.
For all scenarios and indications, we assumed a market penetration rate of 45% in the first year after registration and 90% thereafter.17
Results
Results of list prices and cost-effectiveness publications
HTA reports and BIA dossiers were found for Germany,18-20 France,21-23 the United Kingdom,24-26 the Netherlands,27-29 and Spain. Only in German publications, list prices were stated for all indications. In France, all prices were marked as confidential, and in the United Kingdom, prices were stated for all indications treated with tisagenlecleucel. The UK price for axicabtagene ciloleucel was marked confidential, that is, it was concealed in the report. Dutch prices were available for axicabtagene ciloleucel and tisagenlecleucel.
For Italy and Spain, HTA/BIA reports were not publicly available. List prices for these countries were retrieved from documents of the Italian Medicines Agency (AIFA),30,31 and the Spanish Ministry of Health.32,33 Table 1 presents an overview of all list prices.
Table 1.
Overview of List Prices.
| Country | List Price (Excl. VAT) | ||
|---|---|---|---|
| Axicabtagene ciloleucel (Yescarta) | Tisagenlecleucel (Kymriah) | ||
| DLBCL | pALL | DLBCL | |
| France | 350 000 EUR | 320 000 EUR | 320 000 EUR |
| Germany | 327 000 EUR | 320 000 EUR | 320 000 EUR |
| Italy | 327 000 EUR | 300 000 EUR | 300 000 EUR |
| The Netherlands | 327 000 EUR | 320 000 EUR | 320 000 EUR |
| Spain | 327 000 EUR | 320 000 EUR | 320 000 EUR |
| United Kingdom | 318 773 EUR (300 000 GBP) | 318 773 EUR (282 000 GBP) | 318 773 EUR (282 000 GBP) |
DLBCL = diffuse large B-cell lymphoma; EUR = Euro; GBP = Pound sterling; pALL = pediatric acute lymphoblastic leukemia; VAT = value added tax.
The initial literature search detected 9 CEA,34-41 and the search for gray literature found 3 HTA reports42-44 and 1 report from an Evidence Review Group (ERG) for a National Institute for Health and Care Excellence, Single technology appraisal.37 Two publications were added following the update search.38,39 The publication by Walton et al37 presented results from the Incremental Cost-Effectiveness Ratio (ICER) HTA report and is therefore included in the following summary instead of the HTA report. Most studies focused on pALL patients, while 3 publications40,41,45 studied relapsed/refractory (r/r) DLBCL as indication. The ICER report42 presented results for both r/r pALL and r/r DLBCL.
The results are summarized in Table 2.
Table 2.
Overview of Cost-effectiveness Analysis Publications.
| References | Indication, Treatment | Base-case Settings | Scenario Analysis | Total Costs | Total Effects in QALYs | ICER: CAR T-cell vs |
|---|---|---|---|---|---|---|
| Lin et al34 | pALL, tisagenlecleucel | Perspective: healthcare | Yes, 5-y relapse-free survival rates (ie, 40%-0%) | (2017 USD) | Clo-M: 3.12 | (USD/QALY) |
| Horizon: lifetime | Clo-M: 314 000 | Clo-C: 3.52 | Best-case scenario (40% 5-y relapse-free survival rate) | |||
| Discount rate (costs/effects): 3%/3% | Clo-C: 374 000 | Blina: 3.57 | Clo-M: 61 315, Clo-C: 43 103, Blina: 50 712 | |||
| Blina: 282 000 | CAR T-cell: 8.74 | |||||
| CAR T-cell: 599 000 | ||||||
| Whittington et al35 | pALL, tisagenlecleucel | Perspective: healthcare | Yes, other discount rates, different survival curve fitting, future healthcare cost (included/not included | (2017 USD) | Clo-M: 2.10 | (USD/QALY) |
| Horizon: lifetime | Clo-M: 337 256 | CAR T-cell: 9.28 | Base-case scenario: 46 000 | |||
| Discount rate (costs/effects): 3%/3% | CAR T-cell: 666 754 | |||||
| Sarkar et al36 | pALL, tisagenlecleucel | Perspective: healthcare | No | (2017 USD) | Clo-C: 8.58 | (USD/QALY) |
| Horizon: lifetime | Clo-C: 440 600 | CAR T-cell: 16.76 | Payer perspective: 64 600 | |||
| Discount rate (costs/effects): 3%/3% | CAR T-cell: 968 800 | |||||
| Walton et al37 | pALL, tisagenlecleucel | Perspective: NA | No | (2017 GBP) | Salvage chemo: NA | (GBP/QALY) |
| Horizon: lifetime | Salvage chemo: NA | Blin: NA | Deterministic: Salvage chemo 45 397, Blina: 27 732 | |||
| Discount rate (costs/effects): 3.5%/3.5% | Blina: NA | CAR T-cell: NA | ||||
| CAR T-cell: NA | ||||||
| Furzer et al38 | pALL, tisagenlecleucel | Perspective: Public insurer | Yes, long-term cure rates varying between 10% and 40% | (2018 USD) | Comparator: 5.05 | (USD/QALY) |
| Horizon: 60 y | Comparator (combination of chemo and HSCT): 86 597 | Optimistic scenario: 53 933 | ||||
| Discount rate (costs/effects): 1.5%/1.5% | CAR T-cell: 442 098 | CAR T-cell: 14.90 | ||||
| Thielen et al39 | pALL, tisagenlecleucel | Perspective: Societal | Yes, different perspectives, shorter plateau phase, different time horizons, alternative standardized mortality rate input, vial sharing assumed, longer duration of IVIG administration, different parametric extrapolation models resulting in different cure rates | (2018 EUR) | Clo-M: 0.74 | (EUR/QALY) |
| Horizon: lifetime | Clo-M: 160 803 | Clo-C: 1.70 | Base-case | |||
| Discount rate (costs/effects): 4%/1.5% | Clo-C: 193 920 | Blina: 2.25 | Clo-M: 36 378 EUR/QALY | |||
| BlinaL 267 259 | CAR T-cell: 11.26 | Clo-C: 31 052 EUR/QALY | ||||
| CAR T-cell: 552 679 | Blina: 31 682 EUR/QALY | |||||
| Roth et al40 | DLBCL, axicabtagene ciloleucel | Perspective: healthcare | Yes, patients in long-term remission experience 10% or 20% lower survival compared with age-matched US general population | (2018 USD) | R-DHAP: 1.13 | (USD/QALY) |
| Horizon: lifetime | R-DHAP: 172 737 | CAR T-cell: 7.67 | Base-case scenario: 58 146 | |||
| Discount rate (costs/effects): 3%/3% | CAR T-cell: 552 921 | |||||
| Lin et al45 | DLBCL, tisagenlecleucel, and axicabtagene ciloleucel | Perspective: healthcare | Yes, PFS at 5 y: Axicabtagene: 40% to 20% and tisagenlecleucel: 35% to 15% | (2018 USD) | Combination: R-DHAP, R-GDP, R-GEMOX, R-ICE, SCT: 1.78 | (USD/QALY) |
| Horizon: lifetime | Combination: R-DHAP, R-GDP, R-GEMOX, R-ICE, SCT: 169 000 | Axicabtagene ciloleucel: 5.50 | Axicabtagene ciloleucel vs combination (40% 5-y progression-free survival): 129 570 | |||
| Discount rate (costs/effects): 3%/3% | Axicabtagene ciloleucel: 651 000 | Tisagenlecleucel: 3.92 | Tisagenlecleucel vs combination (35% 5-y progression-free survival): 168 224 | |||
| Tisagenlecleucel: 529 000 | ||||||
| Whittington et al41 | DLBCL, axicabtagene ciloleucel | Perspective: public payer care | Yes, different extrapolation of OS and PFS curves, different perspectives, different time horizons | (Year not clear USD) | R-DHAP: 3.37 | (USD/QALY) |
| Horizon: lifetime | R-DHAP: 151 200 | CAR T-cell: 9.19 | Public payer perspective, standard parametric: 230 900 | |||
| Discount rate (costs/effects): 3%/3% | CAR T-cell: 554 700 |
CAR T = chimeric antigen receptor T cell; DLBCL = diffuse large B-cell lymphoma; EUR = Euro; GBP = Pound sterling; HSCT = autologous haematopoietic stem cell transplantation; ICER = incremental cost-effectiveness ratio; IVIG = intravenous immunoglobulin; pALL = pediatric acute lymphoblastic leukemia; NA = not available; OS = overall survival; PFS = progression-free survival; QALY = quality-adjusted life year; R-DHAP = rituximab dexamethasone cytarabine cisplatin; R-GDP = rituximab gemcitabine dexamethasone cisplatin; R-GEMOX = rituximab gemcitabine oxaliplatin; R-ICE = rituximab ifosfamide carboplatin etoposide; SCT = stem cell transplantation; USD = United States dollar.
Table 3.
Cost Components and Resource Use of Pre- and Post-CAR T-cell Therapy.
| Item | Type | Value in EUR |
|---|---|---|
| Leukapheresis and cryopreservation | Costs | 4947 |
| CAR T-cell administration + lymphodepletion | Costs | 15 033 |
| ICU stay (per day) | Costs | 1444 |
| Hospital stay at hematology/oncology ward (per day) | Costs | 628 |
| IVIG (per dose) | Costs | 2032 |
| Tocilizumab (per event) | Costs | 1483 |
| Treatment of febrile neutropenia (per event) | Costs | 4953 |
| Treatment of anemia (average costs per event, incl. transfusion) | Costs | 2961 |
| Treatment of thrombocytopenia (per event) | Costs | 2417 |
| Oncologist/hematologist (per visit) | Costs | 145 |
| Neurologist (per visit) | Costs | 103 |
| MRI scan (per scan) | Costs | 214 |
| PET-CT scan (per scan) | Costs | 1110 |
| Percentage of patients receiving tocilizumab | Resource use | 60%a |
| Percentage of patients receiving IVIG | Resource use | 24% |
| Assumed average number of days in hospital (including pre- and post-treatment) | Resource use | 14 |
| Assumed average number of ICU days (including pre- and post-treatment) | Resource use | 2 |
| Percentage of patient admitted to ICU | Resource use | 20%a |
| Probability of patients with CRS ≥ 3 | Resource use | 18% |
| Probability of patients with FN | Resource use | 23% |
| Probability of patients with neurological events ≥ 3 | Resource use | 20% |
| Probability of patients with anemia | Resource use | 27% |
| Probability of patients with thrombocytopenia | Resource use | 19% |
| Duration of follow-up (y) | Resource use | 15a |
aBased on clinical experts.
CAR T = chimeric antigen receptor T cell; CRS = cytokine release syndrome; EUR = Euro; FN = febrile neutropenia; ICU = intensive care unit; IVIG = intravenous immunoglobulin; PET-CT = positron emission tomography and computed tomography.
Identification of future CAR T-cell indications
The search on clinicaltrials.gov resulted in a total of 246 studies, of which most were attributed to non-Hodgkin’s lymphoma (n = 97), followed by ALL (n = 84), multiple myeloma (MM) (n = 38), chronic lymphocytic leukemia (CLL) (n = 22), acute myeloid leukemia (AML) (n = 19), and others (n = 35). Several studies addressed multiple indications and targets. The 3 most studied target antigens were CD19 (n = 161), followed by B-cell maturation antigen (n = 19) and CD22 (n = 20).
The clinical experts expected that mantle cell lymphoma (MCL), follicular lymphoma (FL), MM, CLL, and AML, would be the first indications for which CAR T-cell therapy would become available in the near future. Based on phases of the clinical trials and clinical expert opinion, we estimated market entry of CAR T-cell therapies for MCL in 2021. For the indications of MM, CLL, and FL market entry was estimated for the year 2022. Finally, it was expected that CART T-cell therapies for AML would be available in 2025.
Estimation of the eligible patient population
Reported yearly IRs varied not only across but also within countries. Although targeting the same indication, HTA/BIA reports for DLBCL stated different yearly incidences for the same indication and hence different numbers of eligible patients within the same country. For our analysis, we used country averages for pALL and DLBCL in case more than 1 estimate was available. IRs for MCL, FL, AML, MM, and CLL were taken from ECIS (see Appendix VII, http://links.lww.com/HS/A130).
The proportion of eligible patients for CAR T-cell therapies were available for pALL in Germany, France, and the Netherlands and varied between 6% (France) and 11% (Germany). For DLBCL, the proportions were known for Germany, France, the United Kingdom, and the Netherlands, varying between 12% (France) and 22% (United Kingdom). Missing data for these indications (ie, pALL and DLBCL) in all other countries were imputed with the mean proportion from countries with available data (see for details Appendix VII, http://links.lww.com/HS/A130).
To estimate the number of patients for the different cancer subtypes from Globocan, we used United States (US) figures since European data were not available. As proportions were not available from 1 single source, data for pALL were based on the Surveillance, Epidemiology, and End Results (SEER) Program of the US National Cancer Institute.46 Most recent data for ALL and CLL were taken from the 2019 facts and figures sheet published by the American Cancer Society,7 and DLBCL estimates were based on Li et al.9 Proportions of MCL and FL patients from non-Hodgkin lymphoma were taken from Sandoval-Sus et al8 and Cerhan,3 respectively.
For the period 2019–2029, we estimated a total average of 103 750 patients being eligible for CAR T-cell therapies, ranging from 95 954 patients (Eurostat forecast) to 111 545 patients (Globocan forecast) for the indications pALL, DLBCL, MCL, FL, AML, CLL, and MM.
Expenditure estimation of CAR T-cell therapies for current and selected future indications per scenario
Scenario 1: Estimation based on list prices
Multiplying costs for CAR T-cell therapies with the number of eligible patients in the former EU-5 and the Netherlands resulted in average cumulative expenditures varying between 1.4 billion EUR for the Netherlands and 6.7 billion EUR for Germany. Cumulative expenditure estimates in our base-case for pALL, DLBCL, MCL, FL, AML, CLL, and MM for all included countries from 2019 to 2029 were on average 0.8 billion EUR, 13.7 billion EUR, 2.3. billion EUR, 6.4 billion EUR, 1.2 billion EUR, 0.9 billion EUR, and 3.5 billion EUR, respectively (total average: 28.6 billion EUR). Figure 2 depicts the yearly average forecasted expenditure per country for scenario 1 across all indications.
Figure 2.

Total average costs per country in scenario 1 (all indications).
Scenario 2: Total CAR T-cell therapy costs, including pre- and post-costs
Resource use and prices for the cost items considered for scenario 2 could partly be retrieved from sources for the Netherlands, the United Kingdom, Germany, and France (see Table 4 for an overview of the average resource use and cost prices). The additional costs for CAR T-cell therapy amounted to 50 359 EUR for each patient receiving CAR T-cell therapy, with a substantial amount necessary for lymphodepletion and administering CAR T-cells, namely 26 615 EUR. In Table 4, these costs are shown. Cumulative expenditure estimates in our base-case for pALL, DLBCL, MCL, FL, AML, CLL, and MM for all included countries from 2019 to 2029 were on average 0.9 billion EUR, 15.8 billion EUR, 2.5 billion EUR, 7.4 billion EUR, 1.4 billion EUR, 1.1 billion EUR, and 4 billion EUR, respectively (total average: 32.9 billion EUR).
Table 4.
Average Total Costs Pre- and Post-CAR T-cell Administration in Former EU-5 and NL.
| Item | Value in EUR |
|---|---|
| Average cost of care pre-CAR T-cell administration | 7147 |
| Average cost lymphodepletion and administering CAR-T | 26 615 |
| Average cost of care managing AEs | 10 524 |
| Average cost of follow-up | 6074 |
| Total cost of pre- and post-CAR-T care | 50 359 |
AE = adverse event; CAR T = chimeric antigen receptor T cell; EU-5 = France, Germany, Spain, Italy and the United Kingdom; EUR = Euro; NL = the Netherlands.
Multiplying the total costs of pre- and post-CAR T-cell care with the number of eligible patients per indication and country resulted in total cumulative expenditures between 7.7 billion EUR (Germany) and 1.6 billion EUR (Netherlands). Figure 3 depicts average forecasted costs (all indications) per country for scenario 2.
Figure 3.
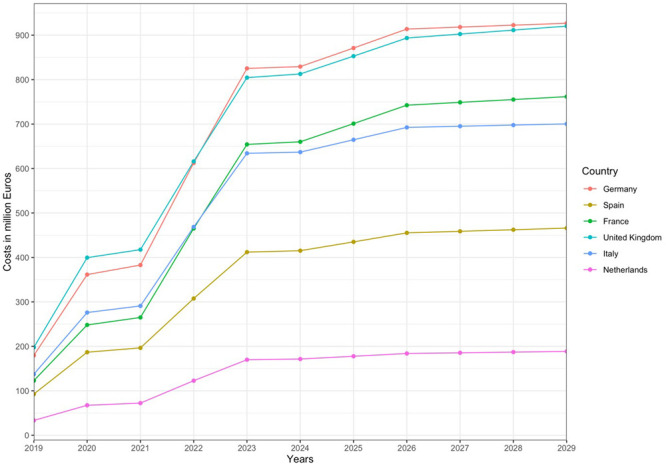
Total average costs per country in scenario 2 (all indications).
Scenario 3: Incremental costs of introducing CAR T-cell therapy
Of all CEA studies reviewed, the total average incremental costs of CAR T-cell therapies when compared to care as usual were 276 086 EUR and 328 727 EUR for patients with pALL and DLBCL, respectively. Cumulative expenditure estimates in our base-case for pALL, DLBCL, MCL, FL, AML, CLL, and MM for all included countries from 2019 to 2029 were on average 0.7 billion EUR, 13.8 billion EUR, 2.3 billion EUR, 6.5 billion EUR, 1.2 billion EUR, 0.9 billion EUR, and 3.5 billion EUR, respectively (total average: 28.9 billion EUR).
Figure 4 depicts the average expenditure across all countries and indications of all 3 scenarios. The upper and lower bounds are the estimates based on the Globocan and Eurostat approach, respectively.
Figure 4.
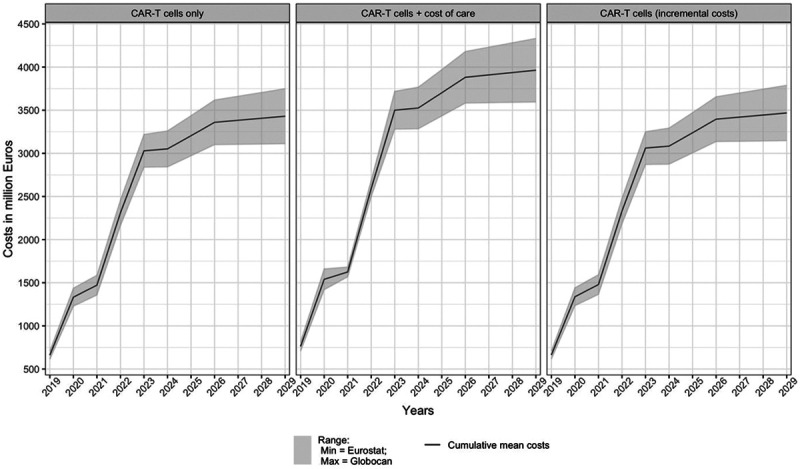
Expenditure forecast per scenario (all countries and indications). CAR-T = chimeric antigen receptor T cell.
Discussion
In our analysis, we estimated future expenditures associated with CAR T-cell therapies for a set of hematological indications in 6 European member states between 2019 and 2029. The average cumulative costs in all 6 countries for all included indications were estimated at 28.5 billion EUR (scenario 1) with a steady increase in yearly average costs across the time range studied. Average yearly costs increased in a stepwise manner, which can be explained by the assumed drug penetration rate and predicted new indication launches. For the year 2019, we assumed penetration rates of 45% for current CAR T-cell therapies for DLBCL and pALL. This penetration rate peaks in 2020 (90%) and remains stable thereafter. We assumed new product launches for MCL for the year 2021 and FL, MM, and CLL in the year 2022. Even with an initial penetration rate of 45% for the first year of the product launch, this is a major cost driver that more than doubled the yearly average costs. Finally, the product launch for AML was estimated for the year 2025 and is responsible for another stepwise increase in predicted yearly cumulative costs.
It seems obvious that new product launches have a considerable impact on any expenditure. Therefore, the methodology for estimating the expenditure of these launches is crucial. However, there is no reliable way of knowing at what time exactly new CAR T-cell therapies will be available for treatment. For product launches of future indications, we used data on available clinical trials on clinicaltrials.gov and estimated their future availability based on the time between the trial start date and the published date of the respective HTA reports for tisagenlecleucel and axicabtagene ciloleucel. In case several trials were currently running, we selected studies from the biggest sponsor in terms of market capitalization. However, this approach neglects the possibility of failing trials that would not lead to market access of a drug, the possibility of smaller companies to be the first to receive market access for their drug, or the possibility of postponing market access due to internal decisions. Therefore, we validated our findings with clinical experts who suggested CAR T-cell therapy launches in the years 2021 and 2022 for MCL and FL, respectively.
The eligible patient population for CAR T-cell therapies in the different EU member states was based on the population projection by Eurostat4 with fixed IRs, and the incidence projection from Globocan, over the period 2019 to 2029. Both strategies were used to congregate an average patient population. The factual eligible patient population could deviate from our projection due to unforeseen events and assumptions. Our assumptions and results were validated by clinical experts, but forecasts are sensitive to changes in outcomes and business strategies. Besides, future clinical pathways may also change, accommodating for new treatments that are currently in the pipeline. Advancements in other immunotherapies and targeted therapies could affect future uptake of CAR T-cell therapies as well. Currently, available CAR T-cell therapies (ie, tisagenlecleucel and axicabtagene ciloleucel) are being investigated for the second-line treatment of patients with DLBCL (NCT03570892, NCT03391466), which will make those therapies available to an even larger patient population. Moreover, lisocabtagene maraleucel is also being investigated in a second-line setting for patients with B-cell non-Hodgkin lymphomas (NCT03575351). If CAR T-cell therapies are utilized in second-line settings, this would considerably increase the eligible patient population.
Besides the uncertainties regarding the number of patients eligible for CAR T-cell therapies, the price of the therapy itself is associated with a high degree of uncertainty. For our analysis, we used list prices whenever available. However, actual prices for CAR T-cell therapies are mostly subject to confidential negotiations. Hence, the actual price per country is unknown. For our analysis, the price of CAR T-cell therapies for future indications was assumed to be 323 500 EUR per treatment, based on an average of the known list prices for DLBCL patients. This estimation could be inaccurate due to existing and future competing treatment options. Moreover, clinical experts already reported a new and lower price for tisagenlecleucel in Germany of 275 000 EUR per treatment. Such a price reduction could be the result of the 2 CAR T-cell therapies (ie, tisagenlecleucel and axicabtagene ciloleucel) currently competing for DLBCL. The expected approval of lisocabtagene maraleucel47 (Celgene) could drive up competition even more. To allow competition with the 2 existing CAR T-cell therapies could lead to an even further reduction in prices. Per contra, Celgene might price lisocabtagene maraleucel higher than its competitor considering the possibility of being best-in-class.48 Yet another scenario that could affect prices of CAR T-cell therapies is the point-of-care production within hospitals, leaving healthcare payers with only the manufacturing costs. Specialized hospitals in several countries are exploring the possibility to make their own CAR T-cell treatments in the future. We have asked clinical experts whether they think it would be an option for lowering the price and improving the access to CAR T-cells for patients. In Germany and the Netherlands, the probability was estimated above 50% and the cost of own production was estimated to be 50 000-70 000 EUR per CAR T-cell treatment. This means that 1 treatment may cost approximately 80 000 EUR (including pre- and post-care costs) instead of 375 000 EUR. In the literature, the manufacturing costs have been estimated at 65 000 USD.49 Moreover, companies such as Cellectis or Servier are currently working on the development of allogeneic CAR T-cell therapies (NCT03190278, NCT02808442). These off-the-shelf CAR T-cell therapies could be manufactured in batches instead of on-demand, resulting in economies of scale, and possibly lower cost for healthcare payers. Lastly, the possibility of in vivo reprogramming of T cells, to, for example, be active against CD19 positive cells, could potentially reduce treatment costs by circumventing ex vivo manufacturing of T cells.50
While the price for CAR T-cell therapies may be subject to changes, the cost of care associated with CAR T-cell therapy could also decrease over time. This may be due to possible reductions in side-effects or different AE profiles with future CAR T-cell therapies. Likewise, our forecasted incremental costs may differ. Our estimates are based on relatively scarce cost-effectiveness data on both tisagenlecleucel and axicabtagene ciloleucel. For future indications, we assumed an average of the known costs. However, according to clinical experts, the incremental costs associated with CAR T-cell therapies for MM could be much lower when compared with DLBCL, for instance. This may be caused by the chronic nature of MM and its current high costs for the standard of care, which could be redundant after CAR T-cell therapies.
Other cell and gene therapies that have regenerative or curative potential are currently being developed for various indications.51 The limited duration of clinical trials is coincidentally accompanied by uncertainty in long-term effects. Moreover, the possibility to cure patients with a single administration presents a new challenge for pricing and reimbursement of these therapies.52 Current pricing of gene therapies ought to reflect expected long-term effects and its curative potential. For instance, Novartis has priced Zolgensma, a gene therapy medication used to treat spinal muscular atrophy in children less than 2 years old, at approximately 1.887 million EUR (2.125 million USD), which makes it the most expensive drug currently available.53 Spark Therapeutics Inc’s Luxturna gene therapy for patients with inherited retinal disease was priced at approximately 754 817 EUR (850 000 USD) for both eyes. One aspect these cell and gene therapies share is their high prices, which are often justified by significant treatment effects. However, long-term efficacy results are not yet available, and some patients may need subsequent CAR T-cell therapies or allogeneic stem cell transplantation. In addition, some patients might need additional (other) gene therapies in the future. It remains unclear who should bear the financial risk stemming from the uncertainty in the clinical value. Consequently, reimbursement decision-makers in many EU member states seem to be reluctant in applying “standard” reimbursement criteria to CAR T-cell therapies.
Several EU member states and the United Kingdom adopted various pricing and reimbursement schemes. While France and the United Kingdom opted for coverage with evidence development schemes, both Italy and Spain negotiated outcomes-based staged payment agreements. Outcomes-based rebates were negotiated in Germany, and in Austria, different cost-sharing agreements are in place, varying between provinces. In the Netherlands tisagenlecleucel for pALL, patients are reimbursed through standard criteria, since its estimated budget impact was found to be relatively low (approximately 10 children per year were estimated to be eligible). Axicabtagene ciloleucel for DLBCL patients, on the other hand, was placed in the so-called “lock” for 421 days before being reimbursed. The different reimbursement schemes for the former EU-5 are analyzed and discussed in depth elsewhere.54
At the 2020 EHA/European Society for Blood and Marrow Transplantation CAR T-cell Meeting in Sitges, manufacturers signaled a willingness to further cooperate with payers reaching reimbursement agreements. Presented options were discounts of list prices, price-volume agreements, outcome-based agreements based on patient-level outcomes, value-based agreements based on additional clinical evidence, or a price by indication. Despite this, CAR T-cell therapies are still not affordable for many countries.
We limited our study to the former EU-5 and the Netherlands, all of which are already reimbursing CAR T-cell therapies. However, difficulties regarding reimbursement are even greater in Eastern Europe, resulting in many patients currently lacking access to these promising treatments.
The future market of CAR T-cell therapies has been studied previously, although not with a specific focus on hematology-oncology.
The decision resources group (DRG), for instance, published a report on CAR T-cell therapies in the pipeline and a forecast snapshot. Without revealing the employed methodology, the DRG estimated the CAR T-cell therapy market at approximately 1.5 billion EUR (1.7 billion USD) by 2026 for the hematological malignancies. It is not clear whether these figures ought to reflect the United States, European, or a global market. Our estimation exceeds the DRG figures by far, but since the methodological approaches cannot be compared, it remains open which forecasted aspects differ.
Another study estimated 114 737 cumulative treated patients in the United States between the years 2019 and 2029 for all hematological cancers.17 This is relatively close to our estimate considering a fundamentally different methodological approach and the inclusion of different cancer types. In terms of costs, Quinn et al17 mention a range between 11.1 billion EUR (12.5 billion USD) and 88.8 billion EUR (100 billion USD) for all hematological cancers. Our estimates fall within this range. However, it needs to be noted that although the US population is comparable to the studied population in terms of size (US population is roughly 96% of the former EU-5 + Netherlands), costs for CAR T-cell therapies are generally higher in the United States.
Finally, we conclude that, although current and future CAR T-cell therapies seem promising in hematological cancers, with the current price-setting, the financial burden on healthcare systems in former EU-5 and the Netherlands is considerable. Some European countries are struggling with associated costs of pre- and post-care for CAR T-cell therapies as these costs are reimbursed insufficiently. Further, the pricing of CAR T-cell therapies is high, and it can be expected that new and commercial CAR T-cell therapies will be in a similar price range. Combined with the expected expansion of indications, the financial burden on healthcare systems will increase substantially with direct effects on patient access to these new treatment options. Specialized hospitals could produce CAR T-cell treatments themselves in the future at lower costs, which could drive procurement costs down. Stimulating this development may contribute to better patient access, but future research and development from manufacturers must be guaranteed.
Sources of funding
This project was funded with an unrestricted grant by the European Haematology Association (EHA).
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
RH and FWT contributed equally to this study.
Supplemental digital content is available for this article.
References
- 1.Jürgens B, Clarke NS. Evolution of CAR T-cell immunotherapy in terms of patenting activity. Nat Biotechnol. 2019; 37:370–375. [DOI] [PubMed] [Google Scholar]
- 2.Charrot S, Hallam S. CAR-T cells: future perspectives. HemaSphere. 2019; 3:e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerhan JR. Epidemiology of follicular lymphoma. Hematol Oncol Clin North Am. 2020; 34:631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eurostat. People in the EU - Population Projections. 2017. Available at: https://ec.europa.eu/eurostat/databrowser/view/proj_19np/default/table?lang=en. Accessed January 20, 2021.
- 5.European Commission. European Cancer Information System. Estimates of Cancer Incidence and Mortality in 2018, for All Countries. 2018. Available at: https://ecis.jrc.ec.europa.eu. Accessed January 20, 2021.
- 6.GCO & IARC. Globocan. Cancer Tomorrow. 2020. Available at: http://gco.iarc.fr/tomorrow/home. Accessed January 20, 2021.
- 7.American Cancer Society. Cancer Facts & Figures 2019. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html#. Accessed January 20, 2021.
- 8.Sandoval-Sus JD, Sotomayor EM, Shah BD. Mantle cell lymphoma: contemporary diagnostic and treatment perspectives in the age of personalized medicine. Hematol Oncol Stem Cell Ther. 2017; 10:99–115. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018; 50:74–87. [DOI] [PubMed] [Google Scholar]
- 10.Bouwmans C, Janssen J, Huijgens P, et al. Costs of haematological adverse events in chronic myeloid leukaemia patients: a retrospective cost analysis of the treatment of anaemia, neutropenia and thrombocytopenia in patients with chronic myeloid leukaemia. J Med Econ. 2009; 12:164–169. [DOI] [PubMed] [Google Scholar]
- 11.Hakkaart-van Roijen L, van der Linden N, Bouwamans C, et al. Kostenhandleiding: methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Bijlage 1. 2016. [Google Scholar]
- 12.Weycker D, Danel A, Marciniak A, et al. Economic costs of chemotherapy-induced febrile neutropenia among patients with non-Hodgkin’s lymphoma in European and Australian clinical practice. BMC Cancer. 2012; 12:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bock JO, Brettschneider C, Seidl H, et al. Ermittlung standardisierter Bewertungssätze aus gesellschaftlicher Perspektive für die gesundheitsökonomische evaluation. Gesundheitswesen. 2014; 77:53–61. [DOI] [PubMed] [Google Scholar]
- 14.Högy B, Keinecke HO, Borte M. Pharmacoeconomic evaluation of immunoglobulin treatment in patients with antibody deficiencies from the perspective of the German statutory health insurance. Eur J Health Econ. 2005; 6:24–29. [DOI] [PubMed] [Google Scholar]
- 15.Lefrant JY, Garrigues B, Pribil C, et al. The daily cost of ICU patients: a micro-costing study in 23 French intensive care units. Anaesth Crit Care Pain Med. 2015; 34:151–157. [DOI] [PubMed] [Google Scholar]
- 16.Tan S, Martin J, Pezzi A, et al. Microcosting study of ICU costs in three European countries. Crit Care. 2008; 12:P526. [Google Scholar]
- 17.Quinn C, Young C, Thomas J, et al. Estimating the clinical pipeline of cell and gene therapies and their potential economic impact on the US healthcare system. Value Health. 2019; 22:621–626. [DOI] [PubMed] [Google Scholar]
- 18.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Tisagenlecleucel (akute lymphatische B-ZellLeukämie). Bewertung gemäß § 35a Abs. 1 Satz 11 SGB V. Nr. 689. 2018. Available at: https://www.g-ba.de/downloads/92-975-2569/2018-09-15_Bewertung-Therapiekosten-Patientenzahlen-IQWiG_Tisagenlecleucel-ALL-D-376.pdf. Accessed January 20, 2021.
- 19.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Tisagenlecleucel (diffuses großzelliges B-ZellLymphom). Bewertung gemäß § 35a Abs. 1 Satz 11 SGB V. Nr. 689. 2018. Available at: https://www.iqwig.de/de/projekte-ergebnisse/projekte/gesundheitsoekonomie/g18-10-tisagenlecleucel-diffus-grosszelligen-b-zell-lymphom-bewertung-gemaess-35a-abs-1-satz-11-sgb-v.10620.html. Accessed January 20, 2021.
- 20.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Axicabtagen-Ciloleucel (primär mediastinales großzelliges B-Zell-Lymphom) – Bewertung gemäß § 35a Abs. 1 Satz 11 SGB V. Nr. 716. 2019. Available at: https://www.g-ba.de/downloads/92-975-2749/2018-11-01_Bewertung-Therapiekosten-Patientenzahlen-IQWiG_Axicabtagen-Ciloleucel-D-416.pdf. Accessed January 20, 2021.
- 21.Haute Autorité de santé. Kymriah® (Tisagenlecleucel) Lymphome diffus à grandes cellules B. 2018. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2018-12/kymriah_ldgcb_pic_ins_avis3_ct17238.pdf. Accessed January 20, 2021.
- 22.Haute Autorité de santé. Kymriah® (Tisagenlecleucel) Leucémie aigue lymphoblastique. 2018. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2018-12/kymriah_lal_pic_ins_avis3_ct17202.pdf. Accessed January 20, 2021.
- 23.Haute Autorité de santé. YESCARTA® (axicabtagene ciloleucel) Lymphomes diffus à grandes cellules B (LDGCB) et lymphome médiastinal primitif à grandes cellules B (LMPGCB) réfractaire ou en rechute, après au moins deux lignes de traitement systémique. 2019. Available at: https://www.has-sante.fr/upload/docs/application/pdf/2019-04/yescarta_20190225_avis_efficience.pdf. Accessed January 20, 2021.
- 24.National Institute for Health and Care Excellence. Tisagenlecleucel for Treating Relapsed or Refractory B-Cell Acute Lymphoblastic Leukaemia in People Aged Up To 25 Years. Committee Papers. 2018. Available at: https://www.nice.org.uk/guidance/ta554/documents/final-appraisal-determination-document. Accessed January 20, 2021.
- 25.National Institute for Health and Care Excellence. Axicabtagene Ciloleucel for Treating Diffuse Large B-Cell Lymphoma and Primary Mediastinal Large B-Cell Lymphoma After 2 or More Systemic Therapies. Appraisal Consultation Committee Papers. 2019. Available at: https://www.nice.org.uk/guidance/ta559/documents/appraisal-consultation-document. Accessed January 20, 2021.
- 26.National Institute for Health and Care Excellence. Tisagenlecleucel for Treating Relapsed or Refractory Diffuse Large B-Cell Lymphoma After 2 or More Systemic Therapies. 2019. Available at: https://www.nice.org.uk/guidance/ta567/documents/appraisal-consultation-document. Accessed January 20, 2021.
- 27.Zorginstituut Nederland. Pakketadvies axicabtagene ciloleucel (Yescarta®). 2019. Available at: https://www.zorginstituutnederland.nl/publicaties/adviezen/2019/03/07/pakketadvies-sluisgeneesmiddel-axicabtagene-ciloleucel-yescarta. Accessed January 20, 2021.
- 28.Zorginstituut Nederland. Pakketadvies tisagenlecleucel (Kymriah®) [DLBCL]. 2019. Available at: https://www.zorginstituutnederland.nl/publicaties/adviezen/2019/03/07/pakketadvies-sluisgeneesmiddel-tisagenlecleucel-kymriah. Accessed January 20, 2021.
- 29.Zorginstituut Nederland. Pakketadvies tisagenlecleucel (Kymriah®) [pALL]. 2019. Available at: https://www.zorginstituutnederland.nl/publicaties/adviezen/2018/12/18/pakketadvies-sluisgeneesmiddel-tisagenlecleucel-kymriah-voor-de-behandeling-van-b-cel-acute-lymfatische-leukemie-b-cel-all-bij-kinderen-en-jongvolwassenen-tot-25-jaar. Accessed January 20, 2021.
- 30.AIFA. AIFA approva la rimborsabilità della prima terapia CAR-T. 2020. Available at: https://www.aifa.gov.it/-/aifa-approva-la-rimborsabilita-della-prima-terapia-car-t. Accessed January 20, 2021.
- 31.AIFA. Regime di rimborsabilita’ e prezzo del medicinale per uso umano «Yescarta». 2019. Available at: https://www.aifa.gov.it/documents/20142/961234/Determina_DG-1643-2019_Yescarta.pdf/26464c52-5e7b-e97d-74d5-f66f69039fa0. Accessed January 20, 2021.
- 32.Secretaria general de sanidad y consumo. Nota Informativa de la Reunión de la Comisión Interministerial de Precios de los Medicamentos. Sesión 187 de 30 de Noviembre de 2018. 2018. Available at: https://www.mscbs.gob.es/profesionales/farmacia/pdf/NOTA_INFORMATIVA_DE_LA_CIPM_187_web.pdf. Accessed January 20, 2021.
- 33.Secretaria general de sanidad y consumo. Nota Informativa de la Reunión de la Comisión Interministerial de Precios de los Medicamentos. Sesión 189 de 18 de Marzo de 2019. 2019. Available at: https://www.mscbs.gob.es/profesionales/farmacia/pdf/NOTA_INFORMATIVA_DE_LA_CIPM_189_web.pdf. Accessed January 20, 2021.
- 34.Lin JK, Lerman BJ, Barnes JI, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in relapsed or refractory pediatric B-cell acute lymphoblastic leukemia. J Clin Oncol. 2018; 36:3192–3202. [DOI] [PubMed] [Google Scholar]
- 35.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and value of chimeric antigen receptor T-cell therapy for pediatric patients with relapsed or refractory leukemia. JAMA Pediatr. 2018; 172:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar R, Gloude NJ, Murphy JD. Cost-effectiveness of chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2018; 36:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walton M, Sharif S, Simmonds M, et al. Tisagenlecleucel for the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2019; 37:1209–1217. [DOI] [PubMed] [Google Scholar]
- 38.Furzer J, Gupta S, Nathan PC, et al. Cost-effectiveness of tisagenlecleucel vs standard care in high-risk relapsed pediatric acute lymphoblastic leukemia in Canada. JAMA Oncol. 2020; 6:393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thielen FW, van Dongen-Leunis A, Arons AMM, et al. Cost-effectiveness of anti-CD19 chimeric antigen receptor T-cell therapy in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia. A societal view. Eur J Haematol. 2020; 105:203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J Med Econ. 2018; 21:1238–1245. [DOI] [PubMed] [Google Scholar]
- 41.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term survival and cost-effectiveness associated with axicabtagene ciloleucel vs chemotherapy for treatment of B-cell lymphoma. JAMA Netw Open. 2019; 2:e190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Institute for Clinical and Economic Review. Chimeric Antigen Receptor T-cell Therapy for B-cell Cancers: Effectiveness and Value. Final Evidence Report. 2018. Available at: https://collections-nlm-nih-gov.eur.idm.oclc.org/catalog/nlm:nlmuid-101744954-pdf. Accessed January 20, 2021.
- 43.CADTH. Tisagenlecleucel for Acute Lymphoblastic Leukemia: Economic Review Report. 2019; 61. Available at: https://cadth.ca/sites/default/files/pdf/car-t/op0538-tisagenlecleucel-economic-report-pALL-jan2019.pdf. Accessed January 20, 2021. [Google Scholar]
- 44.Hettle R, Corbett M, Hinde S, et al. The assessment and appraisal of regenerative medicines and cell therapy products: an exploration of methods for review, economic evaluation and appraisal. Health Technol Assess. 2017; 21:1–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin JK, Muffly LS, Spinner MA, et al. Cost effectiveness of chimeric antigen receptor T-cell therapy in multiply relapsed or refractory adult large B-cell lymphoma. J Clin Oncol. 2019; 37:2105–2119. [DOI] [PubMed] [Google Scholar]
- 46.Surveillance, Epidemiology, and End Results Program. Acute Lymphocytic Leukemia - Cancer Stat Facts. SEER. 2019. Available at: https://seer.cancer.gov/statfacts/html/alyl.html. Accessed January 20, 2021. [Google Scholar]
- 47.Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol. 2018; 36:7505–7505. [Google Scholar]
- 48.Makita S, Imaizumi K, Kurosawa S, et al. Chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma: opportunities and challenges. Drugs Context. 2019; 8:212567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meghan Luchi KC. Placing Your CAR-T Bets. 2018; 7. Available at: https://www.bcg.com/de-de/publications/2018/placing-your-car-t-bets. Accessed January 20, 2021. [Google Scholar]
- 50.Pfeiffer A, Thalheimer FB, Hartmann S, et al. In vivo generation of human CD19-CAR T cells results in B-cell depletion and signs of cytokine release syndrome. EMBO Mol Med. 2018; 10:e9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abou-El-Enein M, Elsanhoury A, Reinke P. Overcoming challenges facing advanced therapies in the EU market. Cell Stem Cell. 2016; 19:293–297. [DOI] [PubMed] [Google Scholar]
- 52.Orkin SH, Reilly P. Paying for future success in gene therapy. Science. 2016; 352:1059–1061. [DOI] [PubMed] [Google Scholar]
- 53.Novartis. AveXis Announces Innovative Zolgensma® Gene Therapy Access Programs for US Payers and Families. 2019. Available at: https://www.novartis.com/news/media-releases/avexis-announces-innovative-zolgensma-gene-therapy-access-programs-us-payers-and-families. Accessed January 20, 2021.
- 54.Jørgensen J, Hanna E, Kefalas P. Outcomes-based reimbursement for gene therapies in practice: the experience of recently launched CAR-T cell therapies in major European countries. J Mark Access Health Policy. 2020; 8:1715536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


