Abstract
Ravulizumab (Ultomiris®), a humanized monoclonal antibody that inhibits complement protein C5, is indicated for the treatment of atypical haemolytic uraemic syndrome (aHUS) in several countries, including the USA and those of the EU. Ravulizumab has been re-engineered from eculizumab to extend its terminal elimination half-life, resulting in a more convenient maintenance dosage regimen of once every 4–8 weeks compared with once every 2–3 weeks for eculizumab. In single-arm phase 3 trials, ravulizumab resolved thrombotic microangiopathy in 54% and 78% of treatment-naïve adult and paediatric patients with aHUS, respectively, within 26 weeks. Ravulizumab was also effective in patients with postpartum aHUS and paediatric patients who responded to eculizumab and later switched to ravulizumab. Ravulizumab was generally well tolerated, with no unexpected safety events. The most common treatment-related adverse events with ravulizumab in treatment-naïve patients include headache, diarrhoea and vomiting. With its convenient once every 4–8 weeks maintenance regimen, ravulizumab is an important treatment option for aHUS in adult and paediatric patients.
Video abstract (MP4 80317 KB)
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-021-01481-6, which is available to authorized users.
| Digital Features for this Adis Drug Evaluation can be found at https://doi.org/10.6084/m9.figshare.13844921. |
Ravulizumab: clinical considerations in aHUS
| Longer-acting complement C5 inhibitor than eculizumab; offers convenient once every 4–8 weeks maintenance regimen. |
| Resolves thrombotic microangiopathy in adult and paediatric patients. |
| Generally well tolerated, with no unexpected safety events. |
Introduction
Atypical haemolytic uraemic syndrome (aHUS) is a type of thrombotic microangiopathy (TMA) caused by complement dysregulation and is characterized by a triad of thrombocytopenia, microangiopathic haemolytic anaemia and end organ (predominantly kidneys) damage [1–4]. aHUS is a rare disorder, with an estimated annual incidence of 0.23–1.9 per million population among all ages [5]. Familial or acquired (more common than familial) complement abnormalities are found in 40%–60% of patients with aHUS [1, 6–8], with mutations in the CFH, CFHR3, MCP, C3, CFB and CFI genes predisposing to the development of aHUS [2, 8, 9]. DGKE-deficiency is a rare genetic form of aHUS, where the complement pathway is intact.
In the absence of complement inhibitor treatment, aHUS has a poor prognosis [6, 8]. In a French aHUS registry study, 17% of paediatric and 46% of adult patients progressed to end-stage renal disease (ESRD) or death by 1 month after clinical manifestation, with these rates increasing further at 1 year (29% and 56%, respectively) [6]. Adult ESRD rates were lower in a global aHUS registry study (31% and 49% at 1 and 5 year), which may be related to limitations of this study, such as survivor bias and censoring of patients on eculizumab initiation [8]. Extrarenal manifestations, including CNS, cardiovascular, peripheral vascular, pulmonary, gastrointestinal and skeletal muscle complications, are also common in patients with aHUS [6, 8, 9].
Eculizumab, a monoclonal antibody that inhibits complement protein C5, was the first drug therapy approved for the treatment of life-threatening TMA in patients with aHUS [10, 11]. It improves long-term renal and TMA outcomes in patient with aHUS [12]. However, eculizumab requires intravenous infusions every 2–3 weeks, increasing infusion burden and associated adverse events (AEs). To overcome this problem, a longer-acting anti-C5 monoclonal antibody (Ravulizumab; Ultomiris®) was produced by targeted re-engineering of eculizumab (Sect. 2). Ravulizumab has recently been approved for the treatment of patients with aHUS in several countries, including those in the EU [13], USA [14] and Japan [15] (Sect. 6). The standard formulation of ravulizumab is 10 mg/mL; however, the US FDA has also recently approved a 100 mg/mL formulation (based on a phase 2 study in patients with paroxysmal nocturnal haemoglobinuria [16]), which reduces the infusion time by 60–77% [14]. This article reviews the clinical efficacy, safety and tolerability of ravulizumab in patients with aHUS, with a brief overview of its pharmacological properties.
Pharmacodynamic Properties of Ravulizumab
Ravulizumab is a humanized immunoglobulin G2/4K monoclonal antibody that specifically binds to complement protein C5, inhibiting its cleavage to C5a (the proinflammatory anaphylatoxin) and C5b (the initiating subunit of the terminal complement complex), thereby preventing the generation of the terminal complement complex, C5b9 [13, 14]. Thus, ravulizumab blocks terminal complement-mediated inflammation, cell activation and cell lysis [17]. In patients with aHUS, ravulizumab inhibits complement-mediated TMA (Sect. 4). Ravulizumab does not interfere with the early components of complement activation that are essential for antibody opsonisation and immune complex clearance [13].
Ravulizumab was produced by incorporating two structural changes to eculizumab aimed at extending its terminal elimination half-life (t1/2) [18]. Firstly, two histidine substitutions were made in the complementarity-determining regions, which enhanced the dissociation rate of the monoclonal antibody-C5 complex at pH 6.0, abolishing the target-mediated antibody clearance. Secondly, two amino acid substitutions were made in the Fc region, which enhanced the affinity of the antibody to human neonatal Fc receptor [18].
A bodyweight-based ravulizumab dosage regimen resulted in immediate and complete terminal complement inhibition (i.e. serum levels of free C5 < 0.5 µg/mL) by the end of the first infusion and throughout the 26-week treatment period in adult [19] and paediatric [20, 21] patients with aHUS. The extent and duration of the pharmacodynamic response was dependent on exposure to ravulizumab in patients with aHUS [13, 14].
Pharmacokinetic Properties of Ravulizumab
The pharmacokinetics of intravenous ravulizumab are dose-proportional over a dose range of 200–5400 mg [14]. Steady state therapeutic concentrations are reached after the first dose [13]. In patients with aHUS, the mean volume of distribution of ravulizumab at steady state was 5.22 L, mean clearance was 0.08 L/day and the mean t1/2 was 51.8 days [13, 14]. The metabolism and elimination of ravulizumab is expected to be similar to that of endogenous immunoglobulin G [13].
Bodyweight is a clinically significant covariate on ravulizumab pharmacokinetics (exposure is lower in heavier patients), necessitating a bodyweight-based dosage regimen (Table 1) [13, 14]. There are no clinically relevant differences in ravulizumab pharmacokinetics based on sex, age (10 months to 83 years), race, hepatic impairment and renal impairment (including in patients receiving dialysis and those with proteinuria) [13, 14]. In patients with paroxysmal nocturnal haemoglobinuria, serum trough concentrations were generally similar when the formulation was switched from 10 to 100 mg/mL during maintenance phase [16].
Table 1.
Ravulizumab bodyweight-based dosage regimen
| Bodyweight (kg) | Loading dosea (mg) | Maintenance doseb (mg) |
|---|---|---|
| ≥ 5 to <10c | 600 | 300 |
| ≥ 10 to <20 | 600 | 600 |
| ≥ 20 to <30 | 900 | 2100 |
| ≥ 30 to <40 | 1200 | 2700 |
| ≥ 40 to <60 | 2400 | 3000 |
| ≥ 60 to <100 | 2700 | 3300 |
| ≥ 100 | 3000 | 3600 |
The regimen used in clinical trials [19–21] and recommended in product information [13–15]
aFor patients switching from eculizumab, administer 2 weeks after the last eculizumab infusion
bStarting on day 15 and every 8 weeks thereafter in patients weighing ≥ 20 kg or every 4 weeks thereafter in those weighing < 20 kg
cRavulizumab is not approved in the EU for patients weighing < 10 kg
Ravulizumab serum concentrations may be reduced in patients receiving long-term intravenous human immunoglobulins, as they may interfere with the endosomal neonatal Fc receptor recycling mechanisms [13]. While there is no clinical experience, concomitant administration of ravulizumab with plasma exchange or infusion may reduce serum concentrations of ravulizumab [13, 14].
Therapeutic Efficacy of Ravulizumab
The efficacy of ravulizumab was evaluated in two, single-arm, multicentre, 26-week, phase 3 studies in adult (ALXN1210-aHUS-311; hereafter, Study 311) [19] and paediatric (ALXN1210-aHUS-312; hereafter, Study 312) [20, 21] patients with aHUS. Study 311 enrolled patients aged ≥ 18 years (bodyweight ≥ 40 kg) who were naïve to complement inhibitor treatment [19]. Study 312 enrolled patients aged ≤ 18 years of age (bodyweight ≥ 5 kg) who were naïve to complement inhibitor treatment [21], as well as those who had previously received eculizumab [20].
In both studies, treatment-naïve patients had to have active TMA, characterized by thrombocytopenia (platelet count < 150 × 109/L), haemolysis [serum lactate dehydrogenase (LDH) ≥ 1.5 × upper limit of normal (ULN)] and kidney dysfunction (serum creatinine ≥ ULN or required dialysis) [19, 21]. The eculizumab-experienced cohort in Study 312 had to have documented evidence of aHUS and clinical response to eculizumab, as indicated by stable TMA parameters (i.e. platelet count ≥ 150 × 109/L, serum LDH < 1.5 × ULN and estimated glomerular filtration rate (eGFR) > 30 mL/min/1.73 m2) [20]. Patients with TMA because of a ADAMTS13 deficiency, Shiga toxin E. coli-related HUS, Streptococcus pneumoniae-related HUS (in Study 311) or drug exposure-related HUS (in Study 312) were among those excluded [19–21].
Patients received a bodyweight-based ravulizumab regimen, with a loading dose on day 1, followed by maintenance doses once every 4 weeks (in patients weighing < 20 kg) or once every 8 weeks (in patients weighing ≥ 20 kg) [Table 1] for 26 weeks [19–21]. After the 26-week initial evaluation period, patients could continue an extension period (≤ 4.5 years) on maintenance doses. The primary endpoint was complete TMA response (defined in Table 2) during the 26-week initial evaluation period in the full analysis set (FAS) [19–21].
Table 2.
Efficacy of ravulizumab in treatment-naïve patients with atypical haemolytic uremic syndrome in clinical studies
| Outcomes | Adults (n = 56) [19] | Paediatric pts (n = 18) [13, 21] |
|---|---|---|
| Complete TMA responsea (% pts) [95% CI] | 53.6 [39.6–67.5] | 77.8 [52.4–93.6] |
| Platelet count ≥ 150 × 109/Lb (% pts) [95% CI] | 83.9 [73.4–94.4] | 94.4 [72.7–99.9] |
| Lactate dehydrogenase ≤ 246 U/Lb (% pts) [95% CI] | 76.8 [64.8–88.7] | 88.9 [65.3–98.6] |
| Serum creatinine ≥ 25% ↑ from BLb (% pts) [95% CI] | 58.9 [45.2–72.7] | 83.3 [58.6–96.4] |
| Haematologic normalizationc (% pts) [95% CI] | 73.2 [60.7–85.7] | 88.9 [NA] |
| Haemoglobin ≥ 20 g/L (% pts) [95% CI] | 71.4 [58.8–84.2] | 88.9 [65.3–98.6] |
| Median changes from BL at day 183 (BL) | ||
| Platelet count (× 109/L) ↑ | 125.0 (95.3) | 247.0 (51.3) |
| Lactate dehydrogenase (U/L) ↓ | 310.8 (508) | 1851.5 (1963.0) |
| Estimated glomerular filtration rate (mL/min/1.73 m2) ↑ | 29.0 (10) | 80.0 (22.0) |
| Haemoglobin (g/L) ↑ | 35.0 (85) | NA (74.3) |
BL baseline, NA not available, pts patients, TMA thrombotic microangiopathy, ↑ increase, ↓ decrease
aPrimary endpoint, assessed through week 26; patients had to meet criteria for all TMA components concurrently, and each criterion had to be met at two separate assessments obtained ≥ 28 days apart and at any measurement in between
bComponent of complete TAM response
cPlatelet count ≥ 150 × 109/L plus lactate dehydrogenase ≤ 246 U/L
In Adult Patients
The FAS in Study 311 included 56 patients (median age 40.1 years) of whom 66.1% were women, including 14.3% in the immediate postpartum [19]. At baseline, the majority of patients had extrarenal symptoms (92.9%), pretreatment plasma exchange or infusion (82.8%) and stage 5 chronic kidney disease (71.4%). At study entry, 14.3% of patients had received a kidney transplant, 51.8% required dialysis and 48.2% had been in intensive care for a median 10.1 days [19].
In Study 311, 54% of patients achieved a complete TMA response during the initial evaluation period (Table 2); the median time to complete TMA response was 86.0 days [19]. Substantial improvements in platelet count was seen on day 8 (first assessment timepoint) and in LDH and eGFR on day 29. In addition to the complete TMA response components, the majority of patients showed a haemoglobin response (≥ 20 g/L) on day 183, with substantial improvement seen on day 57. The TMA and haemoglobin response rate data are supported by absolute median changes in platelet count, LDH, eGFR and haemoglobin levels from baseline at day 183 (Table 2) [19].
Secondary endpoints (i.e. renal outcomes) lend additional support to the therapeutic effects of ravulizumab [19]. Of 29 patients who required dialysis at baseline, 58.6% were able to discontinue dialysis after a median 30 days’ treatment; of 27 non-dialysis patients at baseline, 77.8% did not require dialysis at the last available follow-up (≥ 183 days). Furthermore, between baseline and day 183, eGFR improved by at least one category in 68.1% of patients [19].
Ravulizumab was similarly effective in patients with pregnancy-related aHUS [22]. In Study 311, 7 of 8 (87.5%) postpartum patients achieved complete TMA response in a median 32 days. All patients achieved haematologic normalization. Five patients were on dialysis at baseline and all were able to discontinue dialysis within 29 days’ treatment; eGFR improved by at least one category in all eight patients by day 183 [22].
Long-term treatment with ravulizumab further improved clinical outcomes [23]. In the extension study, after a median follow-up of 75.6 weeks (median treatment duration 74.1 weeks), an additional four patients (i.e. 60.7% of patients) achieved complete TMA response; 80.4%, 85.7% and 83.9% of patients had haematologic, platelet count and LDH normalization, respectively, and 62.5% had > 25% improvement in serum creatinine levels [23].
Ravulizumab treatment was associated with improved health-related quality of life (HR QOL), as assessed by the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score [19]. A clinically meaningful improvement (≥ 3-point increase from baseline) in this score was observed in 84.1% (n = 48 evaluable) of patients at day 183 [19]. The efficacy of ravulizumab and its effect on the HR QOL tended to improve further over ≥ 52 weeks [23].
In Paediatric Patients
The FAS of treatment-naïve patients in Study 312 included 18 patients [21]. At baseline, the majority of patients were aged 2 to < 12 years (77.8%) and weighed between ≥ 10 and < 20 kg (mean bodyweight 22.2 kg); 72.2% of patients had pretreatment extrarenal symptoms, 33.3% had stage 5 chronic kidney disease and 33.3% required dialysis [21].
In Study 312, 78% of treatment-naïve patients achieved a complete TMA response during the 26-week initial evaluation period (Table 2) [21]. The median time to the response was 30 days and all responses were maintained through the initial evaluation period. Absolute median changes in platelet count, LDH, eGFR and haemoglobin levels from baseline at day 183 are summarized in Table 2. All six patients who required dialysis at baseline were able to discontinue dialysis (five patients did so by day 43) and no patients initiated dialysis during the study. Furthermore, eGFR severity reduced by at least one category in 15 of 17 (88.2%) patients. In the extension study, through week 50, three additional patients achieved a complete TMA response and thus, 94.4% of patients achieved this response. A clinically meaningful improvement from baseline in paediatric FACIT-Fatigue score was seen in all nine evaluable patients at week 26 and week 50 [21].
Study 312 enrolled 10 eculizumab-experienced patients [20]. These patients were a median 12.5 years old (median bodyweight 47.8 kg) and had received eculizumab for 98–1701 days. One patient had prior kidney transplantation because of aHUS. All patients completed the 26-week initial evaluation period and entered into the extension period. In these patients, renal function (eGFR, chronic kidney disease stage), haematological parameters (platelet count, LDH, haemoglobin) and FACIT-Fatigue scores remained stable throughout the initial evaluation period and up to 1 year of extension period [20].
Tolerability of Ravulizumab
Ravulizumab was generally well tolerated in the phase 3 trials in adult [19] and paediatric [20, 21] patients with aHUS, with no unexpected safety events. Immunogenicity of ravulizumab was low and it had no apparent effect on the pharmacology, efficacy or safety of ravulizumab [13, 14].
In Study 311, all adult patients (n = 58) experienced ≥ 1AEs of any grade, with 93.1%, 79.3%, 53.4% and 24.1% of patients reporting AEs of grade 1, 2, 3 and 4 severity, respectively [19]. The most common (incidence ≥ 20%) treatment-emergent AEs were headache (36.2%), diarrhoea (31.0%), vomiting (25.9%), hypertension (22.4%) and nausea (22.4%). Treatment-emergent serious AEs occurred in 51.7% of patients and the most common (incidence ≥ 3%) were hypertension (5.2%), pneumonia (5.2%), malignant hypertension (3.4%), urinary tract infection (3.4%), septic shock (3.4%) and aHUS (3.4%). Treatment-related AEs occurred in 34.5% of patients, most commonly headache, diarrhoea, and vomiting. Three patients (5.2%) discontinued treatment because of AEs (autoimmune haemolytic anaemia, intracranial haemorrhage and immune thrombocytopenic purpura). Four patients died during the study, none due to the study drug [19].
The safety profile of ravulizumab in paediatric patients was generally similar to that in adult patients and it appeared to be similar in paediatric subgroups based on age [13, 14]. In Study 312, all treatment-naïve paediatric patients (n = 21) experienced treatment-emergent AEs, most commonly (incidence ≥ 20%) pyrexia (47.6%), nasopharyngitis (33.3%), diarrhoea (33.3%), headache (33.3%), vomiting (25%), abdominal pain (28.6%), hypertension (28.6%) and cough (23.8%) [21]. Treatment-related AEs occurred in 47.6% of patients, the most common of which were fever, colds, diarrhoea, vomiting and headache. Serious AEs occurred in 66.7% of patients; with the exception of viral gastroenteritis and abdominal pain, which occurred in two patients each, all other serious AEs occurred in one patient each [21]. Where reported, grade ≥ 3 reactions occurred in 6 of 16 (37.5%) patients and included anaemia, vomiting, upper respiratory tract infections, gastroenteritis, viral infections and hypertension [14]. Among 10 eculizumab-experienced patients switching to ravulizumab, two patients each experienced treatment-related AEs (dyspepsia and musculoskeletal pain, both resolved without any changes to study treatment) and treatment-emergent AEs during ravulizumab infusion [20].
Adverse Events of Special Interest
Mild, transient infusion reactions occurred in 4.5% of patients with aHUS receiving ravulizumab and included lower back pain, decreased or increased blood pressure, limb discomfort, drug hypersensitivity and dysgeusia. These reactions are manageable by interrupting the infusion and appropriate supportive treatment [13, 14].
Due to its mechanism of action (i.e. blocking of terminal complement activation), ravulizumab may increase the risk of meningococcal (Neisseria meningitides) infection/sepsis [13–15]. Although not in aHUS studies, serious cases of meningococcal infections have occurred in ravulizumab clinical studies (3 out of 261 patients with paroxysmal nocturnal haemoglobinuria). The US prescribing information for ravulizumab carries a boxed warning regarding the risk of serious meningococcal infections [14]. To mitigate this risk, all patients must be vaccinated against N. meningitides ≥ 2 weeks before starting ravulizumab. If ravulizumab is initiated < 2 weeks after the vaccination, appropriate prophylactic antibiotics should be administered until 2 weeks after vaccination. Vaccination does not completely eliminate the risk of N. meningitides infection in ravulizumab recipients; therefore, patients should be monitored for early signs of meningococcal infection/sepsis and treated with appropriate antibiotics if required [13–15]. Ravulizumab is contraindicated in patients with N. meningitides infection at treatment initiation in the EU [13], USA [14] and Japan [15], as well in patients not currently vaccinated against N. meningitidis [13, 14] unless they receive prophylactic antibiotics until 2 weeks after vaccination [13] or the risks of delaying ravulizumab treatment outweigh the risks of developing a meningococcal infection [14].
Ravulizumab may also increase the risk of infections caused by Neisseria species other than N. meningitides (e.g. disseminated gonococcal infections) and encapsulated bacteria [13]. Ravulizumab should be administered with caution in patients with active systemic infections. Immunization as per local vaccination guidelines is recommended (and patients aged < 18 years must also be vaccinated against Haemophilus influenzae and pneumococcal infections) before initiating ravulizumab treatment [13].
Dosage and Administration of Ravulizumab
Ravulizumab is indicated in the EU for the treatment of patients with aHUS who weigh ≥ 10 kg and are complement inhibitor treatment-naïve or have received eculizumab for ≥ 3 months and have evidence of response to eculizumab [13]. Ravulizumab is also indicated for the treatment of aHUS in the USA (for inhibiting complement-mediated TMA in adults and paediatric patients aged ≥ 1 month) [14] and in Japan (for patients weighing ≥ 5 kg) [15]. The recommended dosage is based on bodyweight and consists of a loading dose followed by maintenance doses (Table 1). Ravulizumab treatment to resolve TMA should be continued for a minimum of 6 months and then it should be individualized [13, 14]. After discontinuing ravulizumab, patients should be monitored for TMA complications and if they develop, restart of ravulizumab treatment should be considered. [13, 14]. Local prescribing information should be consulted for detailed information, including contraindications, warnings and precautions, drug interactions and use in special patient populations.
Place of Ravulizumab in the Management of aHUS
Prior to complement blockade therapy, plasma infusion or plasmapheresis and supportive care was the standard treatment for aHUS. However, plasma therapy does not treat the underlying pathology and is associated with limited clinical benefits, high rates of ESRD, procedural complications and other AEs [1–4]. Furthermore, plasma therapy for post-kidney transplant recurrence of aHUS was not effective in preventing graft loss, although it appeared to improve graft survival if started before transplantation (i.e. prophylactic use) [1].
The emergence of eculizumab as complement blockade therapy has vastly improved clinical outcomes in patients with aHUS, producing a paradigm shift in aHUS treatment [1–4]. Eculizumab vastly improves long-term renal and TMA outcomes in patient with aHUS [12] and prophylactic use of eculizumab is associated with reduced post-kidney transplant aHUS recurrence and prolonged graft survival [24]. With its efficacy firmly established in the clinical and real-world settings, eculizumab is the new gold standard first-line treatment for aHUS (recommendations published before the approval of ravulizumab) [1, 2, 4]. According to the HUS International, children with aHUS should receive eculizumab within 24–48 h of onset or admission into hospital; if eculizumab is not available, plasma exchange should be started [1]. Complement genetic test results are not required prior to starting eculizumab. Children who have started plasma therapy as first-line treatment during the acute phase, including those who fail to respond to this therapy, should be switched to eculizumab when possible. Although effective, the relative place of eculizumab in the management of complement factor H antibody-associated aHUS remains to be determined. Liver transplantation or combined liver and kidney transplantation is the only option to cure aHUS in patients with severe aHUS and in those with mutations of complement factors synthesized in the liver [1]. Given the pharmacological similarity between eculizumab and ravulizumab, the clinical practice recommendations for eculizumab may also apply for ravulizumab.
In phase 3 trials, a less frequent dosage regimen of ravulizumab (a loading dose, then once every 4–8 weeks) resolved TMA in 54% and 78% of adult and paediatric patients with aHUS within 26 weeks (Sect. 4). Of note, these trials used complete TMA response as the primary endpoint, which is considered a stringent outcome that simultaneously measures complete haematologic (normalization of platelet counts and LDH levels) and renal improvements. Ravulizumab was also effective in patients with postpartum aHUS. In paediatric patients who responded to eculizumab and switched to ravulizumab, haematologic and renal parameters remained stable (Sect. 4.2). Ravulizumab was generally well tolerated, with no unexpected safety events (Sect. 5).
Terminal complement blockade inhibits the formation of membrane attack complex [25]. Thus, eculizumab and ravulizumab may increase the risk of infection with encapsulated pathogens, such as N. meningitides (Sect. 5.1). Meningococcal infections did not occur in clinical trials of ravulizumab in patients with aHUS, suggesting a low risk. Local prescribing information for ravulizumab carries a number of strategies to mitigate the risk of meningococcal infections [13–15]. With these strategies in place, ravulizumab can be expected to have a favourable benefit-risk profile in patients with aHUS, a complex disease to treat (Fig. 1).
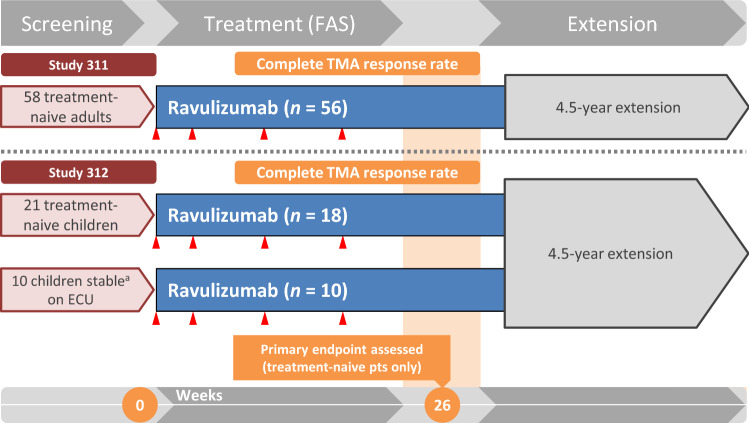
Fig. 1.
Design of the pivotal Study 311 [19] and Study 312 [13, 20] phase 3 clinical trials (efficacy results are reported in the online animated figure). All patients were treated with a bodyweight-based ravulizumab regimen (loading dose on day 1, followed by maintenance doses starting on day 15 and thereafter q8w in patients weighing ≥ 20 kg and q4w in those weighing < 20 kg). Complete TMA response was defined as platelet count ≥ 150 × 109/L, lactate dehydrogenase levels ≤ 246 U/L and serum creatinine ≥ 25% improvement from baseline. ECU eculizumab, FAS full analysis set, pts patients, q4w every 4 weeks, q8w every 8 weeks, TMA thrombotic microangiopathy. aStable TMA parameters
While ravulizumab may have similar efficacy and safety profiles to eculizumab, there are no head-to-head comparative studies in patients with aHUS. An indirect comparison of patient-level data from pivotal trials of ravulizumab (n = 46) and eculizumab (n = 39) indicates that there are no significant between-group differences in platelet count and renal outcomes at 26 weeks, after adjustments for baseline characteristics [26]. While this is the best comparative data available to date, given its indirect nature, this comparison must be interpreted with caution.
The re-engineering of ravulizumab from eculizumab improved the pharmacokinetic properties considerably, with ravulizumab having a 4-fold longer t1/2 (51.8 days) than eculizumab (11.3 days) [18]. Thus, ravulizumab requires less frequent maintenance doses than eculizumab (once every 4–8 weeks vs once every 2–3 weeks), which reduces infusion burden and associated AEs. In addition, ravulizumab is estimated to reduce aggregate duration of therapy (medication preparation time, treatment duration, recovery and travel time) and productivity losses versus eculizumab in Germany, Italy, the UK and the USA [27]. The 100 mg/mL formulation was associated with the lowest aggregate duration of therapy [27].
Considering the high cost of complement blockade treatment, cost is an important determinant of treatment choice [1]. A US cost-minimization analysis estimated that ravulizumab would be 32% less costly than eculizumab in the treatment of aHUS [28]. However, robust cost-utility analyses of complement blockade therapy (including ravulizumab) in aHUS are warranted. In addition, long-term data on renal outcomes and patient survival in ravulizumab recipients are of interest, as life expectancy and the risk of kidney impairment have been shown to be the largest drivers of treatment preference (hence, greatest utility values in pharmacoeconomic modelling) in patients with aHUS [29].
In conclusion, ravulizumab resolves TMA and is well tolerated in adult and paediatric patients with aHUS. It has the same mechanism of action as eculizumab, but with a 4-fold longer duration of action, which substantially reduces the frequency of maintenance doses. Ravulizumab therefore provides an important treatment option for patients with aHUS.
Data Selection Ravulizumab: 70 records identified
| Duplicates removed | 5 |
| Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 20 |
| Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 18 |
| Cited efficacy/tolerability articles | 4 |
| Cited articles not efficacy/tolerability | 23 |
| Search Strategy: EMBASE, MEDLINE and PubMed from 1946 to present. Clinical trial registries/databases and websites were also searched for relevant data. Key words were ravulizumab, Ultomiris, atypical haemolytic uraemic syndrome, aHUS. Records were limited to those in English language. Searches last updated 15 February 2021. | |
Supplementary file1 (MP4 11073 KB)
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
During the peer review process the manufacturer of ravulizumab was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
Yahiya Y. Syed is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, consent to participate, consent to publish, availability of data and material, code availability
Not applicable.
Footnotes
The manuscript was reviewed by: A. Antonelli, Department of Surgical, Medical and Molecular Pathology and Critical Care, University of Pisa, Pisa, Italy; M. Matsumoto, Department of Blood Transfusion Medicine, Nara Medical University, Nara, Japan.
The original version of this article was revised due to a retrospective Open Access order.
Change history
12/7/2021
Video inclusion after abstract
Change history
7/10/2021
A Correction to this paper has been published: 10.1007/s40265-021-01563-5
Change history
4/19/2021
A Correction to this paper has been published: 10.1007/s40265-021-01517-x
References
- 1.Loirat C, Fakhouri F, Ariceta G, et al. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatr Nephrol. 2016;31(1):15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- 2.Fakhouri F, Zuber J, Fremeaux-Bacchi V, et al. Haemolytic uraemic syndrome. Lancet. 2017;390(10095):681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 3.Raina R, Grewal MK, Radhakrishnan Y, et al. Optimal management of atypical hemolytic uremic disease: challenges and solutions. Int J Nephrol Renovasc Dis. 2019;12:183–204. doi: 10.2147/IJNRD.S215370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Organization for Rare Disorders. Atypical hemolytic uremic syndrome. 2016. https://rarediseases.org/. Accessed 5 Nov 2020.
- 5.Yan K, Desai K, Gullapalli L, et al. Epidemiology of atypical hemolytic uremic syndrome: a systematic literature review. Clin Epidemiol. 2020;12:295–305. doi: 10.2147/CLEP.S245642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fremeaux-Bacchi V, Fakhouri F, Garnier A, et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8(4):554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noris M, Caprioli J, Bresin E, et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5(10):1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer F, Ardissino G, Ariceta G, et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94(2):408–418. doi: 10.1016/j.kint.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 9.Raina R, Krishnappa V, Blaha T, et al. Atypical hemolytic-uremic syndrome: an update on pathophysiology, diagnosis, and treatment. Ther Apher Dial. 2019;23(1):4–21. doi: 10.1111/1744-9987.12763. [DOI] [PubMed] [Google Scholar]
- 10.Alexion. SOLIRIS® (eculizumab) injection, for intravenous use: US prescribing information. 2007. https://soliris.net/. Accessed 10 Feb 2021.
- 11.European Medicines Agency. Soliris 300 mg concentrate for solution for infusion: summary of product characteristics. 2007. https://www.ema.europa.eu. Accessed 10 Feb 2021.
- 12.Menne J, Delmas Y, Fakhouri F, et al. Outcomes in patients with atypical hemolytic uremic syndrome treated with eculizumab in a long-term observational study. BMC Nephrol. 2019;20(1):125. doi: 10.1186/s12882-019-1314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency. Ultomiris 300 mg concentrate for solution for infusion: summary of product characteristics. 2020. https://www.ema.europa.eu/. Accessed 9 Nov 2020.
- 14.Alexion. Ultomiris® (ravulizumab-cwvz) injection, for intravenous use: US prescribing information. 2019. https://www.ultomiris.com/. Accessed 9 Nov 2020.
- 15.Alexion. UltomirisⓇ for drip infusion 300 mg: Japanese prescribing information. 2019. https://www.pmda.go.jp/PmdaSearch/iyakuDetail/870056_6399427A1027_1_03#HDR_Warnings. Accessed 15 Feb 2021.
- 16.Röth A, Hill A, Aguzzi R, et al. An interim analysis of a phase 2 study evaluating the efficacy, safety, and pharmacokinetics of intravenous ravulizumab 100 mg/mL formulation in patients with paroxysmal nocturnal hemoglobinuria [abstract no. EP862 plus poster]. In: 25th Annual Congress of the European Hematology Association. 2020.
- 17.European Medicines Agency. Ultomiris: assessment report. 2020. https://www.ema.europa.eu. Accessed 9 Nov 2020.
- 18.Sheridan D, Yu ZX, Zhang Y, et al. Design and preclinical characterization of ALXN1210: a novel anti-C5 antibody with extended duration of action. PLoS ONE. 2018 doi: 10.1371/journal.pone.0195909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondeau E, Scully M, Ariceta G, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in adult patients with atypical hemolytic uremic syndrome naïve to complement inhibitor treatment. Kidney Int. 2020;97(6):1287–1296. doi: 10.1016/j.kint.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Adams B, Aris AM, et al. The long-acting C5 inhibitor, ravulizumab, is efficacious and safe in pediatric patients with atypical hemolytic uremic syndrome previously treated with eculizumab. Ped Neph. 2020 doi: 10.1007/s00467-020-04774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariceta G, Dixon BP, Kim SH, et al. The long-acting C5 inhibitor, ravulizumab, is effective and safe in pediatric patients with atypical hemolytic uremic syndrome naive to complement inhibitor treatment. Kidney Int. 2020 doi: 10.1016/j.kint.2020.10.046. [DOI] [PubMed] [Google Scholar]
- 22.Gäckler A, Dobronravov V, La Manna G, et al. Efficacy and safety of the long-acting C5 inhibitor ravulizumab in patients with postpartum atypical haemolytic uraemic syndrome [abstract no. MO020]. Nephrol Dial Transplant. 2020;35(Suppl 3).
- 23.Barbour T, Scully M, Ariceta G, et al. One-year efficacy and safety of the long acting C5 inhibitor ravulizumab for the treatment of atypical haemolytic uraemic syndrome (aHUS) in adults [abstract no. SO054]. Nephrol Dial Transplant. 2020;35(Suppl 3).
- 24.Zuber J, Frimat M, Caillard S, et al. Use of highly individualized complement blockade has revolutionized clinical outcomes after kidney transplantation and renal epidemiology of atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2019;30(12):2449–2463. doi: 10.1681/ASN.2019040331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence. 2014;5(1):98–126. doi: 10.4161/viru.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rondeau E, Hatswell AJ, Cataland S. Comparative efficacy of ravulizumab and eculizumab in the treatment of atypical hemolytic uremic syndrome: an indirect comparison using clinical trial data [abstract no. PO1851]. In: American Society of Nephrology Annual Meeting. 2020. [DOI] [PMC free article] [PubMed]
- 27.Levy A, Chen P, Tomazos I. Ravulizumab reduces productivity losses versus eculizumab for treating atypical hemolytic uremic syndrome: a cost consequence analysis in four countries. In: ISPOR Europe. 2020.
- 28.Wang Y, Johnston K, Popoff E, et al. A US cost-minimization model comparing lifetime per-patient costs for ravulizumab and eculizumab for the treatment of atypical hemolytic uremic syndrome [abstract no. PRO36] Value Health. 2020;23(Suppl. 1):S335. doi: 10.1016/j.jval.2020.04.1264. [DOI] [PubMed] [Google Scholar]
- 29.Williams K, Aggio D, Chen P, et al. Utility values associated with treatment attributes for atypical hemolytic uremic syndrome: a discrete choice experiment in five countries. In: ISPOR Europe. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.