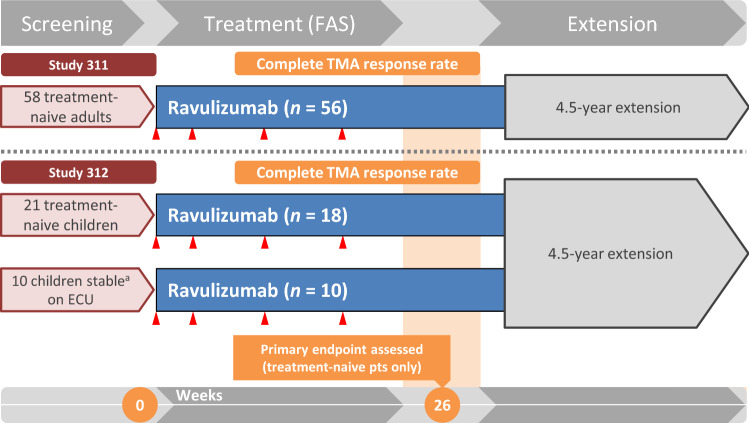
Fig. 1.
Design of the pivotal Study 311 [19] and Study 312 [13, 20] phase 3 clinical trials (efficacy results are reported in the online animated figure). All patients were treated with a bodyweight-based ravulizumab regimen (loading dose on day 1, followed by maintenance doses starting on day 15 and thereafter q8w in patients weighing ≥ 20 kg and q4w in those weighing < 20 kg). Complete TMA response was defined as platelet count ≥ 150 × 109/L, lactate dehydrogenase levels ≤ 246 U/L and serum creatinine ≥ 25% improvement from baseline. ECU eculizumab, FAS full analysis set, pts patients, q4w every 4 weeks, q8w every 8 weeks, TMA thrombotic microangiopathy. aStable TMA parameters