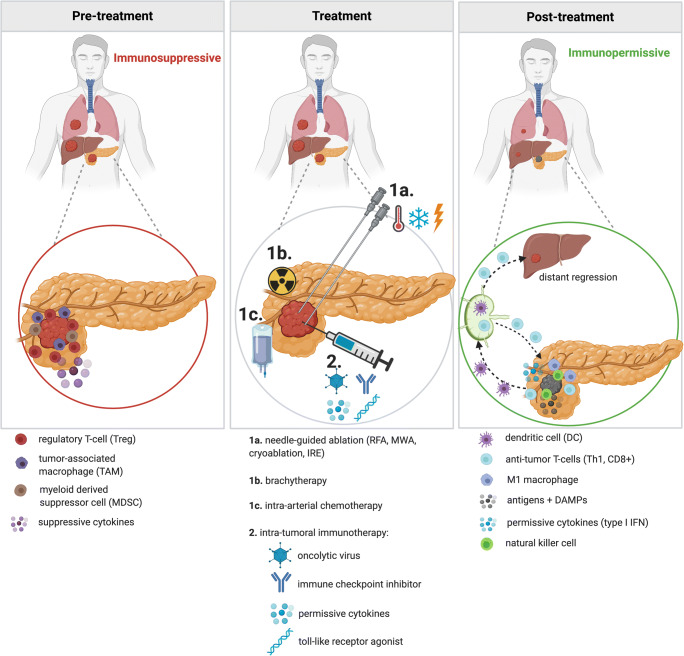
Fig. 1.
Changing immune status upon treatment with local interventional treatment in combination with local immunotherapy. Pre-treatment: pancreatic ductal adenocarcinoma (PDAC) maintains a heavily immunosuppressive environment, established by (among others) regulatory T-cells (Tregs), tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and suppressive cytokines. Treatment: combination treatment with intra-tumoral immune modulation and ablation, brachytherapy, or intra-arterial chemotherapy potentially creates synergism resulting in a durable anti-tumor effect. Immune potentiation combined with local ablation leads to the release of tumor antigens and damage-associated molecular patterns (DAMPs). Subsequently activated dendritic cells (DCs) are now able to capture antigens and migrate towards the draining lymph nodes. Here, antigens are presented to lymphocytes, inducing antigen-specific expansion of effector T-cells, including T-helper-1 cells (Th1) and CD8+ (cytotoxic) T-cells, which will provide systemic anti-tumor immunity. Immune activation will lead to reduced TAMs, Tregs and MDSCs. Post-treatment: The tumor microenvironment demonstrates a more immunopermissive state, comprising of natural killer cells, M1 macrophages, anti-tumor T-cells (Th1 and CD8+), and permissive cytokines such as interferon (IFN). T-cells are also primed to roam the body in search of tumor cells, both at the primary tumor site as well as other locations, possibly resulting in the regression of untreated concomitant (micro)metastases. Figure created with BioRender.com