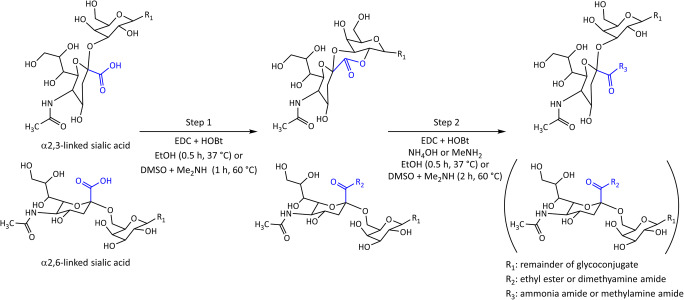
Fig. 1.
Schematic representation of common one-pot in-solution linkage-specific derivatization approaches for sialylated glycoconjugates. In the first step, α2,3-linked sialic acids form a lactone with the subterminal galactose, while α2,6-linked sialic acids are subjected to ethyl esterification or Me2NH (dimethylamine) amidation. In the second step, the lactone undergoes mainly direct aminolysis by NH3 (ammonia) or MeNH2 (methylamine), and the α2,6-sialyllactose derivative remains unchanged