Figure 1.
Overexpression of PAF in lung adenocarcinoma (LUAD) is correlated with poor prognosis
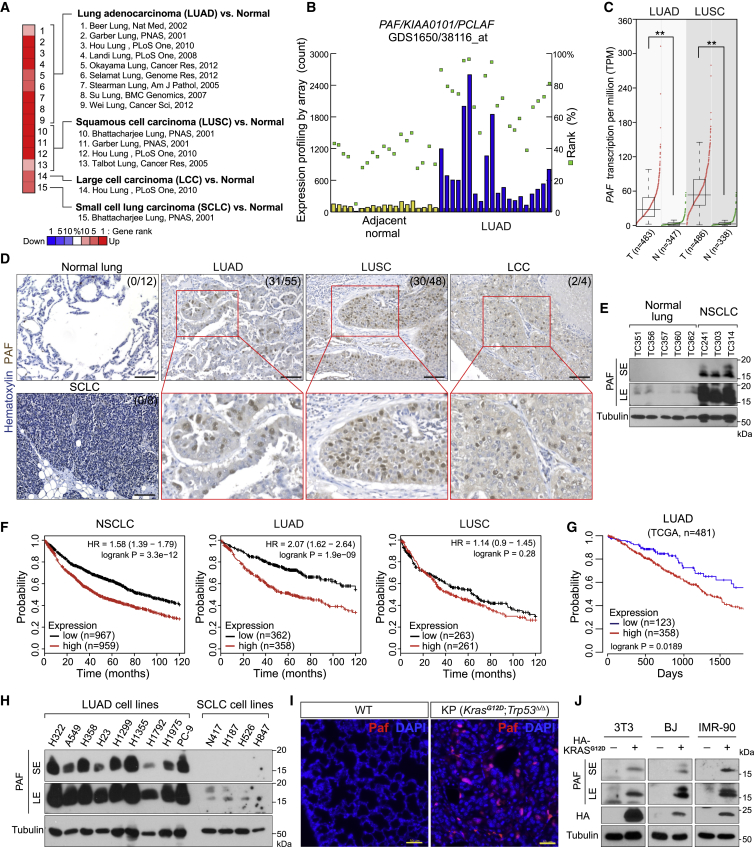
(A) Upregulation of PAF in human lung cancer datasets. Oncomine analysis of PAF expression in lung cancer subtypes (gene rank > top 10%, fold change > 2, p < 0.0001 compared with normal tissues).
(B) Upregulation of PAF in human LUAD. Gene Expression Omnibus (GEO; GDS1650/38116_at) analysis of PAF expression in tumor-adjacent normal (n = 19) and LUAD (n = 20) tissues. Each column indicates PAF expression in individual samples by microarray (signal counts; arbitrary units). Green squares indicate rank order of expression measurements within samples.
(C) Upregulation of PAF in human LUAD and lung squamous cell carcinoma (LUSC). Gene expression in tumor (T) versus normal (N) tissues was analyzed using The Cancer Genome Atlas (TCGA) dataset (GEPIA; boxplots, one-way ANOVA, ∗∗p < 0.01).
(D) Increased levels of PAF protein in human LUAD. Immunohistochemistry (IHC) analysis of human lung cancer tissue microarray samples for PAF expression (anti-PAF antibody). Normal lung (n = 12), LUAD (n = 55), LUSC (n = 48), small cell lung cancer (SCLC) (n = 8), and large cell carcinoma (LCC) (n = 4). PAF protein is localized mainly in the nucleus of tumor cells. Scale bars, 100 μm.
(E) PAF protein expression analyses in human normal lung and non-small cell lung cancer (NSCLC) samples. Five normal lung tissues and three PDX samples (TC241, TC303, and TC314) were examined using immunoblotting for PAF with an anti-PAF antibody. Tubulin served as loading control. SE, short exposure; LE, long exposure.
(F) Increased expression of PAF is associated with poor prognosis of lung cancer patients. Kaplan-Meier (KM) survival curves of patients with NSCLC, LUAD, and LUSC on the basis of PAF expression. The analysis included 1,926 patients with NSCLC, 720 with LUAD, and 524 with LUSC in a publicly available database (KM plotter; Probe = 211713_x_at).
(G) KM survival curves of 481 TCGA patients with LUAD by PAF expression. The lowest quartile was used as the cutoff for dividing PAF-low and PAF-high groups (GEPIA; TCGA).
(H) PAF expression in LUAD cell lines. Whole-cell lysates of LUAD and SCLC cell lines were analyzed using immunoblotting for PAF with an anti-PAF antibody. Tubulin served as loading control.
(I) Paf is expressed in mouse LUAD but not in normal lung tissues. IHC of normal mouse lungs and KrasLSL-G12D/+; Trp53floxed/floxed (KP) lung tumors (3 months after Ad-Cre administration). Scale bars, 50 μm.
(J) Ectopic expression of KRASG12D induces PAF expression. Three non-transformed cell lines (3T3, BJ, and IMR-90) were transiently transfected with plasmids encoding KRASG12D (HA tagged). After 48 h, each cell line was harvested for immunoblotting analysis of endogenous PAF expression. SE, short exposure; LE, long exposure.
Representative images are shown.