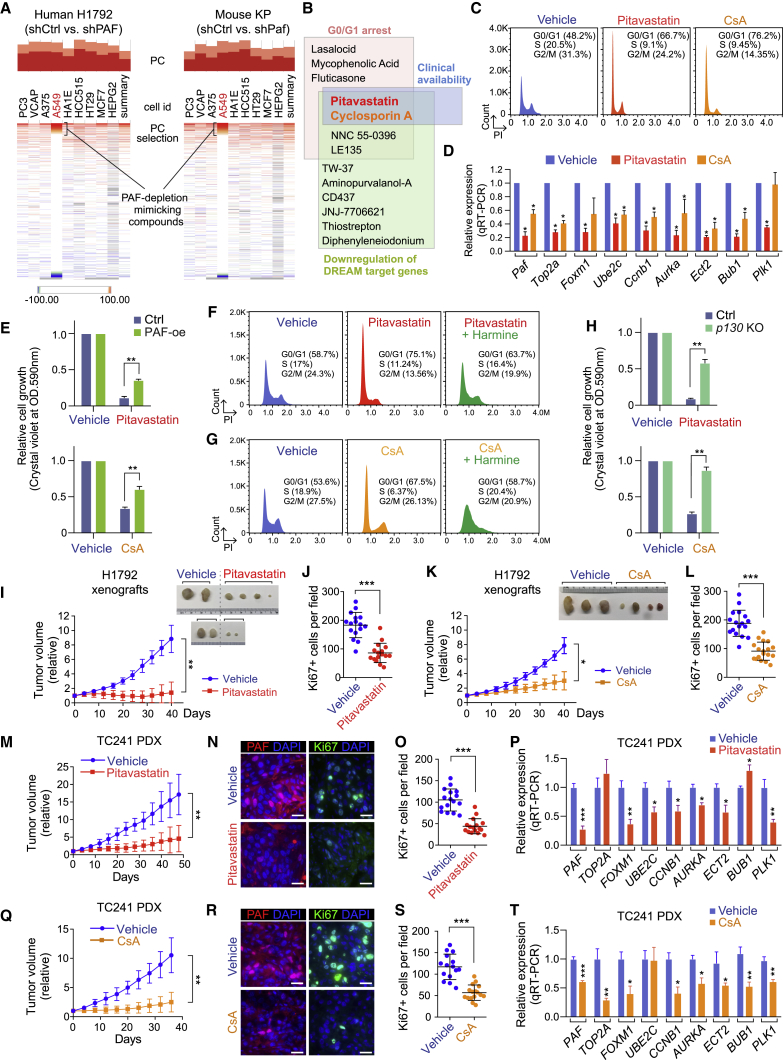
Figure 7.
Suppression of lung tumorigenesis by pharmacological mimicking of PAF-dREAM axis inhibition
(A) Connectivity MAP (CMAP) analysis of PAF-depleted human (H1792) and mouse (KP) transcriptomes (RNA-seq). The chemicals displaying perturbagens with high percentage of connection (PC) were selected for further analysis.
(B) Schematic of the screening for PAF depletion-mimicking drugs.
(C) G0/G1 arrest induced by pitavastatin and CsA. Cell-cycle analysis of control versus pitavastatin-treated (1 μM, 24 h) and CsA-treated (5 μM, 24 h) KP lung cancer cells by FACS.
(D) Downregulation of DREAM target genes by pitavastatin (1 μM, 48 h) and CsA (5 μM, 48 h) in KP cells; qRT-PCR; error bars indicate SEM.
(E) Partial rescue of pitavastatin- or CsA-induced growth inhibition by ectopic expression of PAF. The cell growth of PAF-overexpressing (PAF-oe) A549 cells and parental cells treated with pitavastatin (5 μM) and CsA (5 μM) is shown.
(F–H) Rescue of pitavastatin- or CsA-induced growth inhibition by DREAM complex dissociation. Harmine treatment reduced the G0/G1 arrest induced by (F) pitavastatin or (G) CsA treatment; PI staining-FACS analysis. (H) p130 KO rescued the growth inhibition induced by pitavastatin or CsA treatment. Stably transduced sgCtrl and p130 KO (sgRNA2) A549 cells were used.
(I–L) Growth inhibition of LUAD xenografts by pitavastatin or CsA. Immunocompromised mice were subcutaneously injected with H1792 cells. (I) Relative tumor volumes of vehicle-treated (n = 4) or pitavastatin-treated (n = 6) H1792 xenografts. (J) Reduced cell proliferation of H1792 xenografts by pitavastatin; quantification of Ki67 staining in vehicle- versus pitavastatin-treated H1792 xenografts. (K) Relative tumor volumes of vehicle-treated (n = 3) or CsA-treated (n = 4) H1792 xenografts. (L) Reduced cell proliferation of H1792 xenografts by CsA; quantification of Ki67 staining in vehicle- versus CsA-treated H1792 xenografts. Tumors were monitored for 6 weeks. Images of xenograft tumors at the endpoints are shown, error bars indicate SD. Student’s t test was used in (J) and (L). Two-way ANOVA with Tukey post hoc test was used in (I) and (K).
(M–P) Inhibition of LUAD PDX growth by pitavastatin. (M) Relative tumor volumes of TC241 PDXs treated with vehicle (n = 5) or pitavastatin (n = 5, 10 mg/kg); error bars indicate SD. Two-way ANOVA with Tukey post hoc test. (N and O) Reduced PAF expression and cell proliferation of PDX by pitavastatin. (N) Representative images of PAF and Ki67 immunostaining and (O) quantification of Ki67 in vehicle- versus pitavastatin-treated TC241 PDXs; error bars indicate SD. (P) Downregulation of DREAM target gene expression by pitavastatin; qRT-PCR (n = 3); error bars indicate SEM.
(Q–T) Inhibition of LUAD PDX growth by CsA. (Q) Relative tumor volumes of TC241 PDXs treated with vehicle (n = 6) or CsA (n = 6, 10 mg/kg); error bars indicate SD. Two-way ANOVA with Tukey post hoc test. (R and S) Reduced PAF expression and cell proliferation in PDXs by CsA. (R) Representative images of PAF and Ki67 immunostaining and (S) quantification of Ki67 in the vehicle- versus CsA-treated TC241 PDXs; error bars indicate SD. (T) Downregulation of DREAM target gene expression by CsA; qRT-PCR (n = 3); error bars indicate SEM.
Representative images are shown. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.