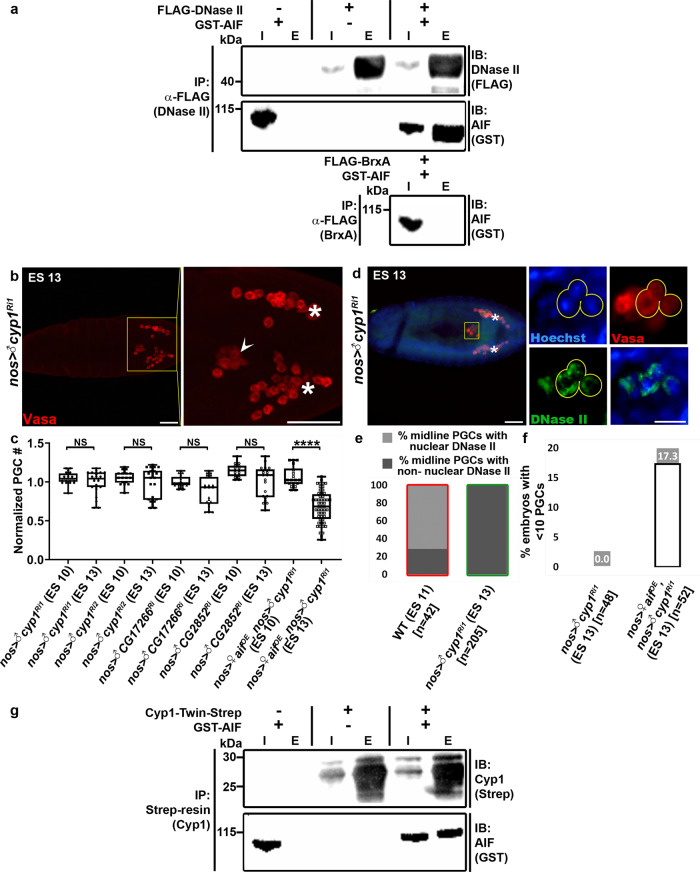
Fig. 8. Interplay between AIF, CypA, and DNase II.
a AIF physically associates with DNase II in vitro. Recombinant DNase II and AIF (tagged with FLAG and GST, respectively) were expressed alone or together in E. coli. AIF was pulled down by immunoprecipitation of DNase II with anti-FLAG antibodies, and the presence of AIF was confirmed using anti-GST antibodies. As a negative control, FLAG-tagged BrxA (a Bacillus cereus protein) was similarly expressed with GST-AIF and pulled down with anti-FLAG antibodies. Both the immunoprecipitates (E, elution) and the corresponding preincubated lysates (I, input) were analyzed by immunoblotting (IB). Note the potent binding of GST-AIF to FLAG-DNase II but not to FLAG-BrxA or to the anti-FLAG beads. b, c The Drosophila cypA orthologs are involved in PGC death. Shown is a representative image of an ES 13 embryo with PGC-specific knockdown of the closest cypA ortholog, cyp1 (b), stained to visualize the PGCs (Vasa; red) and presented as in Fig. 1b. An arrowhead pointing at ectopically surviving midline PGCs. Asterisks indicate gonadal PGCs. Scale bars, 50 μm. The corresponding quantifications of cyp1 knockdown embryos, and embryos with knockdowns in the other cypA orthologs are shown in (c). All data points, including outliers, were presented in box plot format where the minimum is the lowest data point represented by the lower whisker bound, the maximum is the highest data point represented by the upper whisker bound, and the center is the median. The lower box bound is the median of the lower half of the dataset while the upper box bound is the median of the upper half of the dataset. Each dot corresponds to the number of PGCs in a single embryo to reflect n number, where n = number of examined biologically independent embryos. ****p < 0.0001; NS, non-significant; Student’s t-test, one-sided distribution. d, e Nuclear translocation of DNase II is blocked in ectopically surviving midline PGCs in cyp1 knockdown embryos. Shown is a representative image of an ES 13 embryo with PGC-specific cyp1 knockdown (d) stained and presented as in Fig. 3k, l. Asterisks indicate gonadal PGCs. Scale bars, 50 µm. Note the non-nuclear DNase II localization in the ectopically surviving midline PGCs (yellow circles). Scale bars, 10 µm. e Quantification of the percentage of midline PGCs with nuclear and non-nuclear DNase II localization for embryos of the indicated genotypes and ES. Green column outline indicates that the cells being counted are living PGCs, red column outline indicates that the cells being counted are dying PGCs. n number is shown in brackets where n = number of examined PGCs. f Quantification of the percentage of ES 13 embryos with less than 10 PGCs following PGC-specific OE of aif in embryos with PGC-specific cyp1 knockdown. The percent value is indicated above each column. n number is shown in brackets where n = number of examined biologically independent embryos. Note that while OE of aif restored PGC death in the cyp1 knockdown embryos (c), in about 17% of the embryos it caused excessive PGC death (f). Since OE of aif in an otherwise WT background does not affect PGC death levels (Fig. 7b), this finding suggests that the relative Cyp1 levels may modulate the AIF potency to promote cell death. g AIF physically associates with Cyp1 in vitro. Recombinant Cyp1 and AIF (tagged with Twin-Strep and GST, respectively) were expressed alone or together in E. coli. AIF was pulled down by co-purification of Cyp1 with Strep-Tactin, and the presence of AIF was confirmed using anti-GST antibodies. Both the immunoprecipitates (E, elution) and the corresponding preincubated lysates (I, input) were analyzed by immunoblotting (IB). Note the potent binding of GST-AIF to Cyp1-Twin-Strep but not to Strep-Tactin resin.