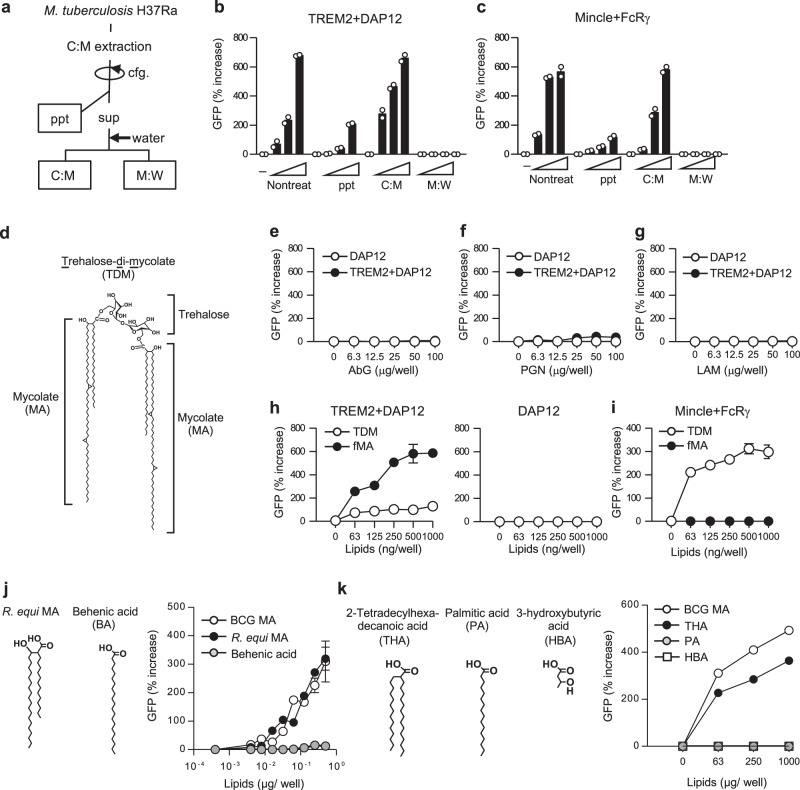
Fig. 2. TREM2 recognizes MAs.
a Schematic diagram of solvent-based de-lipidation and fractionation of Mtb H37Ra. Bacteria were de-lipidated by the treatment with chloroform/methanol. After centrifugation (cfg.), soluble extracts (sup) were mixed with water and separated into the lipid-soluble (C:M) and the water-soluble methanol:water (M:W) fractions. b, c NFAT-GFP reporter cells expressing TREM2 + DAP12 (b) or Mincle + FcRγ (c) were stimulated for 24 h with either untreated or de-lipidated bacteria (ppt) or plate-coated C:M or M:W fractions indicated in a, followed by analysis of GFP fluorescence by flow cytometry. d Chemical structure of TDM. The structure of α-mycolate-containing TDM is shown. e–g NFAT-GFP reporter cells expressing TREM2 + DAP12 or DAP12 alone were stimulated with the indicated amounts of ABG (e), PGN (f), and LAM (g) for 24 h, followed by analysis of GFP fluorescence by flow cytometry. Data are presented as percent increase over control values of unstimulated cells. h, i NFAT-GFP reporter cells expressing TREM2 + DAP12 or DAP12 only (h) or Mincle + FcRγ (i) were stimulated with the indicated amounts of TDM or fMA, and analyzed as described in e–g. j, k NFAT-GFP reporter cells expressing TREM2 + DAP12 were stimulated with the indicated amounts of BCG MA, R. equi MA, or BA (j) or THA, PA, or HBA (k) and analyzed as described in e–g. Data are presented as the mean ± SEM of duplicate (b, c, e–i, j) or triplicate (k) assays and representative of three independent experiments. Source data are provided as a Source data file.