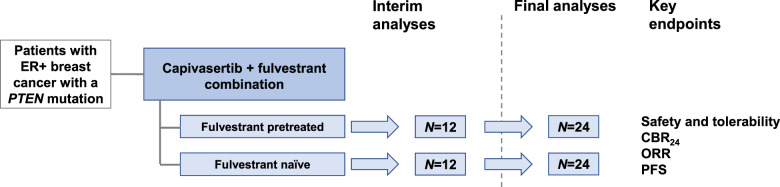
Fig. 3. Study design of the PTEN-mutant breast cancer cohort.
Up to 24 patients in each cohort. Interim analyses were carried out after 12 patients were followed up for 24 weeks or withdrawn from the study. Subsequent patients were recruited only if sufficient antitumor activity (assessed by CBR24) was observed at the interim analyses. CBR24 clinical benefit rate at 24 weeks, ER+ estrogen-receptor-positive, ORR objective response rate, PFS progression-free survival.