Abstract
The successful development of COVID-19 vaccines within an unprecedented short time needs to be followed by rapid vaccine uptake, in particular, in high-risk populations such as patients with cancer. It is important for the scientific research community and cancer physicians to convey the knowledge behind the COVID-19 vaccine development and contribute to build the required trust on their use.
Vaccines are arguably the most successful interventions in modern medicine. The COVID-19 pandemic, caused by infection with the SARS-CoV2 virus, has placed extraordinary focus on the need for safe and effective vaccines to control this deadly infectious agent. Our biomedical research community has shown the incredible capability to rapidly mobilized many experts around this one challenge, and with the support of government and private sector funding mechanisms, rapidly succeed in delivering a number of successful vaccines in an unprecedented short time. This could not have been accomplished without the significant investment in science by our federal agencies and the accelerated federal policies provided by the NIH, FDA, Centers for Disease Control and Prevention (CDC), and Operation Warp Speed, collaboratively bringing tremendous resources to bear on this global challenge. In less than 1 calendar year, we have gone from identification of the causative agent of disease to mass production of multiple vaccines capable of eliciting immunologic protection and limiting the spread of this virus. The truly remarkable pace of this scientific effort is unprecedented in the history of vaccines and medicine in general. A key early goal will be to vaccinate not only frontline health care providers, first responders, and essential workers, but also our susceptible patients with cancer who experience disproportionately poor outcomes of COVID-19 (1), including in some cases long-term viral shedding (2). Thus, ensuring high vaccine uptake in oncology patients is a high priority. This rapid development of SARS-CoV2 vaccines, with all their early promise has also raised some questions. How could we have developed vaccines so quickly when other vaccines against HIV and most cancers remain elusive goals? Can such rapidly developed vaccines be safe? And, if we can do it in less than 1 year for SARS-CoV2, can it be done this quickly for other diseases?
These are important questions that we as scientists and clinicians should be able to answer for our patients and the nonscientists we meet. Indeed, the apparent rapid development of SARS-CoV2 vaccines tells only part of the story. There are three important points to make: (i) research leading to SARS-CoV2 vaccines did not begin in January 2020 when the viral RNA sequence was determined, it actually began more than 15 years ago (or even longer) with fundamental basic science on the biology of coronaviruses; (ii) the vaccine platforms that form the backbone for the most advanced of the SARS-CoV2 vaccines were not invented in 2020, but rather had been under development and even already tested in humans, though some on a greater scale than others, and; (iii) decades of investment in understanding the mechanisms of vaccine-induced immunity, protection from viral infections, pathways by which the immune system generates antibody responses to parts of other coronaviruses provided a foundation from which to start against SARS-CoV2 (Fig. 1). There is a saying—the last 10% of a challenging project often takes 90% of the effort, or at least it seems that way. It may seem like the majority of what it has taken to make these promising SARS-CoV2 vaccines occurred in rapidly in the last 12 months. But make no mistake, the tremendous, unprecedented efforts on SARS-CoV2 vaccines this year, stand on a robust, prolonged scientific effort that placed our biomedical community (including key contributions from cancer researchers) in a position to succeed. Without this previous work, we would be very far behind where we are today.
Figure 1.
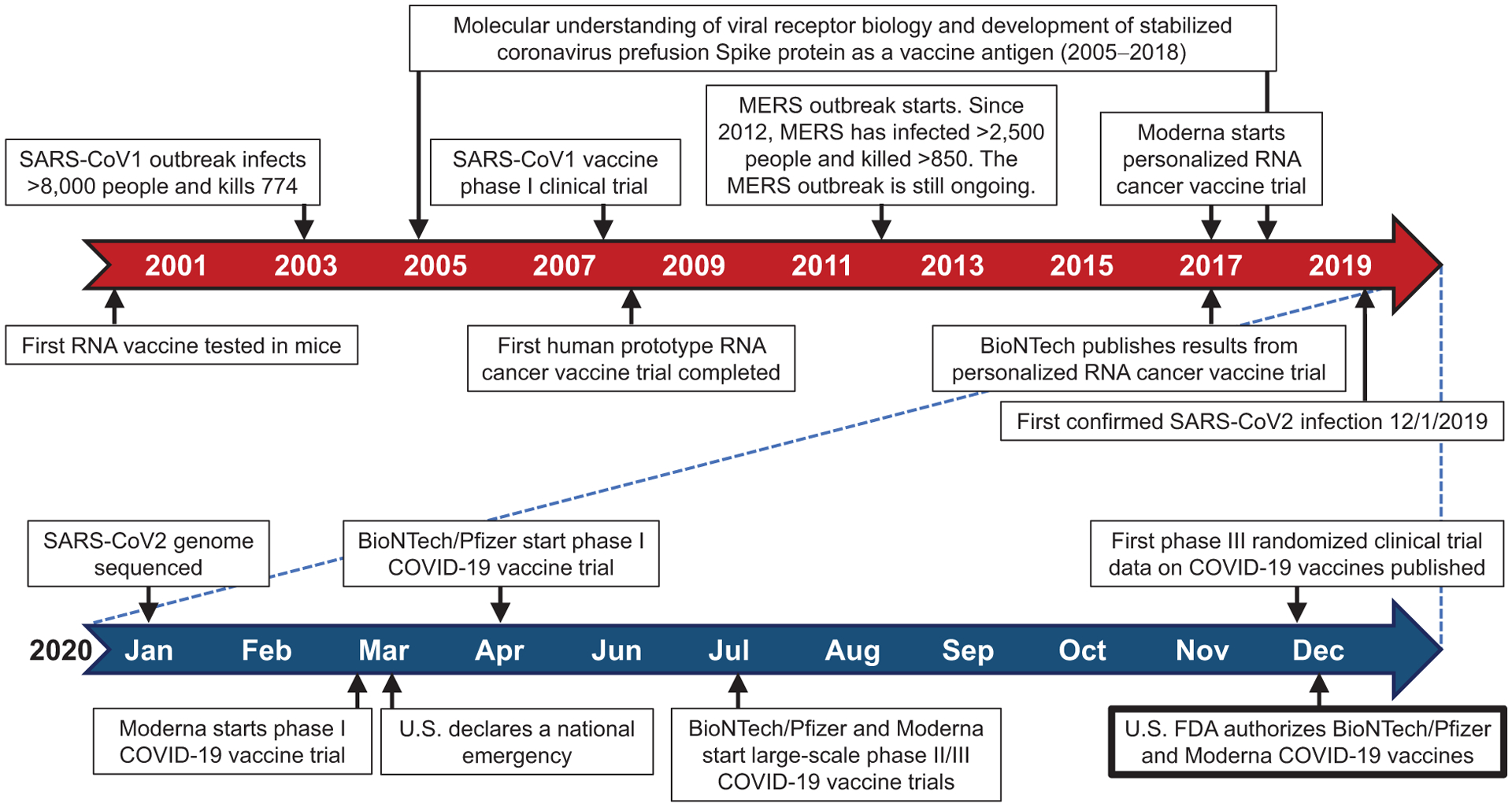
Timeline related to the development of COVID-19 vaccines.
How does this perspective help us answer the questions posed? First, efforts on understanding how to make an effective vaccine to viruses like SARS-CoV2 actually began with efforts on SARS (now SARS-CoV1) starting in approximately 2003 culminating in a phase I human vaccine trial (3) and then efforts on MERS several years later (Fig. 1; ref. 4). This work included key basic science including studying the structure of the Spike protein of other coronaviruses, identifying general principles that were essential for the development of vaccines for, at the time, future unknown coronaviruses (5). Without this work, there would be no SARS-CoV2 vaccine. So, it has actually been an 18-year race to make a SARS-CoV2 vaccine in less than 1 year.
Second, we have considerable safety data for the platforms such as the adenoviral vaccine backbone in development (6), as well the novel mRNA platforms in humans (7). Although the new SARS-CoV2 mRNA vaccines have only been tested in humans since early in 2020 and we do need longer follow-up, all of the leading platforms have an excellent safety track record in humans so far. Cancer research has also played a particular important role alongside work on the virology and immunology of viral infection. The mRNA vaccine platform was developed and tested in humans initially as an experimental cancer vaccine (7, 8) (Fig. 1). To be clinically useful, this application for patients with cancer in clinical trials required an approximately 2-month turnaround to make a personalized cancer vaccine targeting patient-specific tumor mutational neoantigens. This press for time to make a personalized cancer vaccine for each new patient served as an advantage for developing the COVID-19 vaccine, as it took less than 2 months from the time the SARS-CoV2 sequence was known to begin human testing. The versatility and speed of this vaccine platform also ensures that it can be modified to cover new SARS-CoV2 mutant strains. The same way cancer vaccines could be generated to mutational neoantigens in 2 months, new mutations in SARS-CoV2 could be incorporated into future COVID-19 vaccines in a short time.
Third, if we can do it for SARS-CoV2, can we do this for other diseases? This is an important question. The “Manhattan Project” mentality of studying SARS-CoV2 biology and infection this year has led to a remarkable pace of progress. The early investment in understanding the basic science of SARS-CoV1 and MERS created a critical foundation. We have mobilized science in academia, the private sector and government around a unified mission. The answer to the third question may be that the urgency caused by a global pandemic generated the will to do remarkable things with the biomedical research engine of this country. Indeed, the government spending on biomedical research, largely (though not entirely) through the NIH, CDC, and FDA provides arguably one of the best returns on investment for our society. Unfortunately, this same investment on a SARS-CoV2 vaccine in 1 year equals about the same government investment in all serious diseases over 5 or more years.
At a time when there are limiting supplies of highly effective COVID-19 vaccines, the field is faced with the critical task of prioritizing their use. Vulnerable patients with cancer should be among the early prioritized populations given the disproportionate impact of disease in these patients. The available data on patients with cancer and COVID-19 indicates a doubling of case fatality rates compared with subjects with COVID-19 without cancer (1). These data highlight a particular risk of COVID-19 severity and mortality in patients with hematologic malignancies and lung cancer undergoing active therapy. The severe COVID-19 risks in these patients with cancer may relate to specific types of treatments impacting the immune system in hematologic malignancy (e.g., B-cell–depleting therapies or immune-ablating chemotherapy) or lung damage from cigarette smoking or cancer itself in the case of lung cancer (9). This increased risk highlights the need to prioritize these patients with hematologic malignancies and lung cancer for COVID-19 vaccination. Although, there remains relatively little data on the safety and effectiveness of SARS-CoV2 vaccination in patients with cancer, the risk of COVID-19 infection as the pandemic continues may necessitate vaccinating such patients in the absence of specific vaccine data for hematologic malignancies and lung cancer. Existing COVID-19 vaccines induce higher antibody levels than natural SARS-CoV2 infection (10) suggesting that even in patients with cancer with weakened immune systems sufficient vaccine immunity may be induced to provide protection from COVID-19. Furthermore, prioritizing vaccination for patients with cancer may also decrease the risk of these patients becoming chronic carriers and spreading the virus to care providers and family members. A particular practical question on SARS-CoV2 vaccination of patients undergoing active oncologic therapy is the timing. It would be desirable to avoid vaccinating patients during lymphopenia following chemotherapy, but such sequencing may be difficult especially given current COVID-19 vaccine limitations and supply unpredictability. On the other hand, patients on immune checkpoint blockade therapy receive periodic drug infusions that continuously saturate the target for months. Thus, SARS-CoV2 vaccination in these patients would be in the presence of effective blockade of programmed death-1 (PD-1) or CTL antigen 4 (CTLA-4). However, because the mRNA vaccine platform was developed initially for neoantigen cancer vaccines and administered together with immune checkpoint blockade therapy (8), this combination has an adequate safety profile. Furthermore, after years of clinical use, there is no evidence that any viral vaccination has increased toxicities or decreased activity in patients receiving cancer immunotherapies, further supporting the notion that these patients should be recommended to proceed with their COVID-19 vaccination independent of the timing of immune checkpoint blockade therapy (11). However, although there are likely no risks of toxicities with mRNA vaccines delivered in the context of immune checkpoint blockade, whether blocking PD-1 or CTLA-4 has specific impacts on the magnitude or quality of vaccine-induced immunity will need to be evaluated.
In closing, the rapid development of SARS-CoV2 vaccines is a triumph of our modern global biomedical research infrastructure. This success, however, depended critically on decades-long investment in basic science, outstanding public private partnerships, and a highly mobilized research and clinical community. As we deploy these vaccines in the community, we must: continue to study the immunology of the vaccine-induced protection be vigilant about any potential side effects, in particular, in patients with cancer receiving chemotherapy or immunotherapy; study the long-term consequences of disease and we should capitalize on the infrastructure available to rapidly contain this pandemic, and; we must further our understanding of this disease and prepare ourselves for the next biomedical challenges whether from a future pandemic or the eradication of cancer. Thus, the SARS-CoV2 vaccines developed this year appear so far to be remarkably effective and to have an outstanding safety profile thus far. It is a tremendous success story about the investment in basic biomedical research that if replicated for other serious illnesses, could lead to more rapid progress against many diseases, including cancer.
Translational Relevance.
The rapid development of SARS-CoV2 vaccines is a triumph of our modern global biomedical research infrastructure. A key early goal will be to vaccinate not only frontline health care providers, first responders, and essential workers, but also our susceptible patients with cancer who experience disproportionately poor outcomes of COVID-19. We as scientists and clinicians should be prepared to answer questions for our patients regarding the safety and development of COVID-19 vaccines.
Acknowledgments
E.J. Wherry was supported by NIH grants AI105343, AI112521, AI082630, AI201085, AI123539, and AI117950. A. Ribas is supported by NIH grants R35 CA197633, P01 CA244118, and P30 CA016042, Mary Tanner and Maurizio Grimaldi, and The Ressler Family Fund. E.J. Wherry. and A. Ribas are also supported by the Parker Institute for Cancer Immunotherapy.
Authors’ Disclosures
E.J. Wherry reported a patent for U.S. Patent number 10,370,446 licensed to Roche/Genentech; and E.J. Wherry is a founder of Surface Oncology and Arsenal Biosciences. E.M. Jaffee reported grants and nonfinancial support from BMS; personal fees from Genocea, Achilles, DragonFly, CSTONE, Parker Institute, and candel; other from AbMeta; grants from Lustgarten outside the submitted work; in addition, E.M. Jaffee had a patent for GVAX issued. N. Warren reported nonfinancial support and other from Dartmouth College outside the submitted work; and is a full-time employee of the AACR. G. D’Souza reported grants from NIH during the conduct of the study. A. Ribas reported personal fees from Amgen, Chugai, Genentech, Merck, Novartis, Roche, Sanofi, Vedanta, 4C Biomed, Apricity, Arcus, Highlight, Compugen, ImaginAb, MapKure, Merus, Rgenix, Lutris, PACT Pharma, Tango, Advaxis, CytomX, Five Prime, RAPT, Isoplexis, Kite-Gilead; and grants from NCI, Agilent, Bristol-Myers Squibb through Stand Up to Cancer (SU2C), the Melanoma Research Alliance, and the Parker Institute for Cancer Immunotherapy outside the submitted work. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov 2020;11:233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020; 183:1901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin JE, Louder MK, Holman LA, Gordon IJ, Enama ME, Larkin BD, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a phase I clinical trial. Vaccine 2008;26:6338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pallesen J, Wang N, Corbett KS, Wrapp D, Kirchdoerfer RN, Turner HL, et al. Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc Natl Acad Sci U S A 2017;114:E7348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirchdoerfer R, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature 2016;531: 118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris SJ, Sebastian S, Spencer AJ, Gilbert SC. Simian adenoviruses as vaccine vectors. Future Virol 2016;11:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov 2018;17:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020;585:107–12. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Rizvi H, Egger JV, Preeshagul IR, Wolchok JD, Hellmann MD. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov 2020;10:1121–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021;384:80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garassino MC, Ribas A. At the crossroads: COVID-19 and immune checkpoint blockade for cancer. Cancer Immunol Res 2021. January 15 [Epub ahead of print]. doi: 10.1158/2326-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]


