Figure 1.
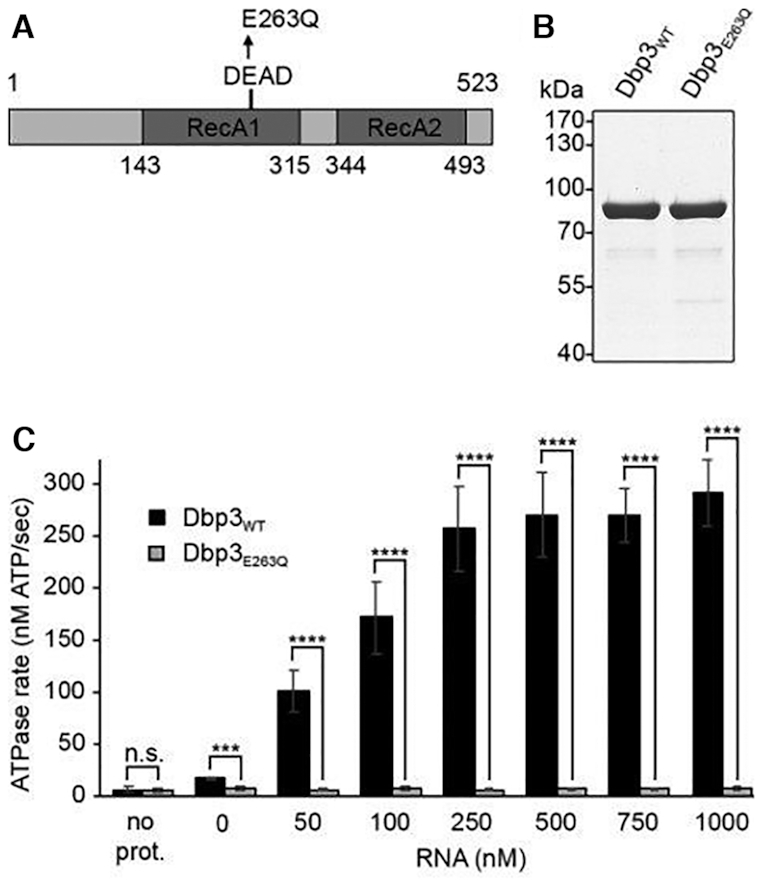
The putative RNA helicase Dbp3 is an RNA-dependent ATPase. (A) Schematic view of Dbp3 showing the amino acid boundaries of the RecA1 and RecA2 domains as well as the amino acid substitution made within the evolutionarily conserved DEAD motif. (B) N-terminally His10-ZZ tagged wild type Dbp3 (Dbp3WT) and an equivalent protein carrying a glutamate to glutamine substitution at position 263 (Dbp3E263Q) were recombinantly expressed in E. coli and purified by Ni2+-affinity chromatography. Purified proteins were separated by SDS-PAGE and visualised by Coomassie staining. (C) In vitro NADH-coupled ATPase assays were used to monitor ATP hydrolysis by Dbp3WT and Dbp3E263Q in the presence of increasing amounts of RNA. A sample containing no protein was included as a control for background hydrolysis of ATP. The data represent the mean of three independent experiments ± standard deviation. ***P< 0.001, ****P< 0.0001, n.s. = non-significant.
